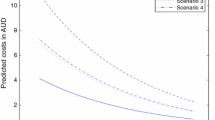
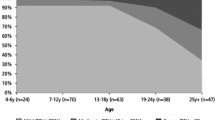
Abstract
Background
Understanding the determinants of cost of cystic fibrosis (CF) care and health outcomes may be useful for financial planning for the delivery of CF services. Registries contain information otherwise unavailable to healthcare activity/cost monitoring systems. We estimated the direct medical cost of CF care using registry data and examined how cost was affected by patient characteristics and CF gene (CF Transmembrane Conductance Regulator [CFTR]) mutation.
Methods
Healthcare resource utilisation data (2008–2012) were obtained for CF patients enrolled with the Irish CF Registry by 2013 from linked registry and national hospitalisation database records. Mean annual hospitalisation and medication per-patient costs were estimated by demographic profile, CFTR mutation, clinical status, and CF co-morbidity, and were presented in 2014 euro values. A mixed-effects regression model was used to examine the effect of demographic, CFTR mutation, and clinical outcomes on the log10 cost of direct medical CF care.
Results
Using 4261 observations from 1100 patients, we found that the median annual total cost per patient increased over the period 2008–2012 from €12,659 to €16,852, inpatient bed-day cost increased from €14,026 to €17,332, and medication cost increased from €5863 to €12,467. Homozygous F508-CFTR mutation (class II) cost was highest and milder mutation (class IV/V) cost was 49% lower. Baseline estimated cost in 2008 for a hypothetical underweight, homozygous F508del-CFTR 6-year-old female without chronic Pseudomonas aeruginosa/Staphylococcus aureus, CF-related diabetes (CFRD) or methicillin-resistant S. aureus (MRSA), and with a poor percent predicted forced expiratory volume in 1 s (ppFEV1) was €10,113, and was €21,082 in a 25-year-old with the same hypothetical profile. Chronic P. aeruginosa infection increased baseline cost by 39%, CF co-morbidity diabetes by 18%, and frequency of pulmonary exacerbation by 15%. Underweight, declining ppFEV1, chronic S. aureus colonisation, and time also influenced cost.
Conclusions
CFTR mutation is an important factor influencing the cost of CF care. Costs differ among cohorts of CF patients eligible to access new and emerging CFTR repair therapies. These findings support the evaluation of outcome-associated cost in CFTR mutation-specific CF patient groups.
Similar content being viewed by others
Notes
Prices of medications reimbursed through these schemes are available from https://www.sspcrs.ie/druglist/pub;JSESSIONID12=lhkZvbKllsPXHh7zzHj7mq-TjUy_mUFHUYZb_unSvOKx3wAegmuD!-295550195!1860876782.
Available from the Healthcare Pricing Office.
References
Cutting GR. Cystic fibrosis genetics: from molecular understanding to clinical application. Nat Rev Genet. 2015;16(1):45–56.
Stephenson AL, Sykes J, Stanojevic S, et al. Survival comparison of patients with cystic fibrosis in canada and the united states: a population-based cohort study. Ann Intern Med. 2017;166(8):537–46.
Grosse SD, Rosenfeld M, Devine OJ, Lai HJ, Farrell P. Potential impact of newborn screening for cystic fibrosis on child survival: a systematic review and analysis. J Pediatr. 2006;149:362–6.
Davis PB. Cystic fibrosis since 1938. Am J Respir Crit Care Med. 2006;173(5):475–82.
Sawicki GS, Sellers DE, Robinson WM. High treatment burden in adults with cystic fibrosis: challenges to disease self-management. J Cyst Fibros. 2009;8(2):91–6.
Smyth AR, Bell SC, Bojcin S, Bryon M, Duff A, Flume P, et al. European Cystic Fibrosis Society standards of care: best practice guidelines. J Cyst Fibros. 2014;13:S23–42.
Gu Y, Garcia-Perez S, Massie J, van Gool K. Cost of care for cystic fibrosis: an investigation of cost determinants using national registry data. Eur J Health Econ. 2015;16(7):709–17.
Heimeshoff M, Hollmeyer H, Schreyogg J, Tiemann O, Staab D. Cost of illness of cystic fibrosis in Germany: results from a large cystic fibrosis centre. Pharmacoeconomics. 2012;30(9):763–77.
Lieu TA, Ray GT, Farmer G, Shay GF. The cost of medical care for patients with cystic fibrosis in a health maintenance organization. Pediatrics. 1999;103(6):e72.
Ouyang L, Grosse SD, Amendah DD, Schechter MS. Healthcare expenditures for privately insured people with cystic fibrosis. Pediatr Pulmonol. 2009;44(10):989–96.
van Gool K, Norman R, Delatycki MB, Hall J, Massie J. Understanding the costs of care for cystic fibrosis: an analysis by age and health state. Value Health. 2013;16(2):345–55.
Briesacher BA, Quittner AL, Fouayzi H, Zhang J, Swensen A. Nationwide trends in the medical care costs of privately insured patients with cystic fibrosis (CF), 2001–2007. Pediatr Pulmonol. 2011;46(8):770–6.
Farrell P, Joffe S, Foley L, Canny GJ, Mayne P, Rosenberg M. Diagnosis of cystic fibrosis in the Republic of Ireland: epidemiology and costs. Ir Med J. 2007;100(8):557–60.
Cystic Fibrosis Foundation Patient Registry. Annual data report 2015. Bethesda: Cystic Fibrosis Foundation; 2016. https://www.cff.org/Research/Researcher-Resources/Patient-Registry/CF-Patient-Registry-Reports/. Accessed 5 July 2017.
Cystic Fibrosis Registry of Ireland. Annual data report 2015. Dublin: CFRI; 2017. https://www.cfri.ie/docs/annual_reports/CFRI2015.pdf. Accessed 5 July 2017.
Drummond M, Sculpher M, Torrance G, O’Brien B, Stoddart G. Methods for the economic evaluation of health care programmes. 3rd ed. Oxford: Oxford University Press; 2005.
Health Information and Quality Authority. A guide to health technology assessment at HIQA. 2016. https://www.hiqa.ie/sites/default/files/2017-01/A-Guide-to-Health-Technology-Assessment.pdf. Accessed 17 Mar 2017.
cftr.info. Classification of CFTR mutations. 2017. http://www.cftr.info/about-cf/cftr-mutations/the-six-classes-of-cftr-defects/. Accessed 16 June 2017.
Koch C, Cuppens H, Rainisio M, Madessani U, Harms H, Hodson M, et al. European Epidemiologic Registry of Cystic Fibrosis (ERCF): comparison of major disease manifestations between patients with different classes of mutations. Pediatr Pulmonol. 2001;31(1):1–12.
Zielenski J. Genotype and phenotype in cystic fibrosis. Respiration. 2000;67(2):117–33.
Johansen HK, Nir M, Hoiby N, Koch C, Schwartz M. Severity of cystic fibrosis in patients homozygous and heterozygous for ∆F508 mutation. Lancet. 1991;337(8742):631–4.
De Boeck K, Zolin A. Year to year change in FEV1 in patients with cystic fibrosis and different mutation classes. J Cyst Fibros. 2017;16(2):239–45.
McKone EF, Goss CH, Aitken ML. CFTR genotype as a predictor of prognosis in cystic fibrosis. Chest. 2006;130(5):1441–7.
Zolin A, McKone EF, van Rens J. ECFS Patient Registry annual data report: 2014 data. European Cystic Fibrosis Society Patient Registry; 2016. https://www.ecfs.eu/sites/default/files/general-content-files/working-groups/ecfs-patient-registry/ECFSPR_Annual%20Report%202014_Nov2016.pdf. Accessed 5 July 2017.
R Development Core Team. R software version 2.10.1. Vienna: R Foundation for Statistical Computing; 2008.
Davies JC, Wainwright CE, Canny GJ, Chilvers MA, Howenstine MS, Munck A, et al. Efficacy and safety of ivacaftor in patients aged 6 to 11 years with cystic fibrosis with a G551D mutation. Am J Respir Crit Care Med. 2013;187(11):1219–25.
Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365(18):1663–72.
Nakagawa S, Schielzeth H. A general and simple method for obtaining RB2 from generalized linear mixed-effects models. Methods Ecol Evol. 2013;4(2):133–42.
Burgel P-R, Bellis G, Olesen HV, Viviani L, Zolin A, Blasi F, et al. Future trends in cystic fibrosis demography in 34 European countries. Eur Respir J. 2015;46(1):133–41.
Jackson AD, Daly L, Jackson AL, Kelleher C, Marshall BC, Quinton HB, et al. Validation and use of a parametric model for projecting cystic fibrosis survivorship beyond observed data: a birth cohort analysis. Thorax. 2011;66(8):674–9.
Rosenfeld M, Davis R, FitzSimmons S, Pepe M, Ramsey B. Gender gap in cystic fibrosis mortality. Am J Epidemiol. 1997;145(9):794–803.
Schreyogg J, Hollmeyer H, Bluemel M, Staab D, Busse R. Hospitalisation costs of cystic fibrosis. Pharmacoeconomics. 2006;24(10):999–1009.
Dewitt EM, Grussemeyer CA, Friedman JY, Dinan MA, Lin L, Schulman KA, et al. Resource use, costs, and utility estimates for patients with cystic fibrosis with mild impairment in lung function: analysis of data collected alongside a 48-week multicenter clinical trial. Value Health. 2012;15(2):277–83.
Johnson JA, Connolly MA, Jacobs P, Montgomery M, Brown NE, Zuberbuhler P. Cost of care for individuals with cystic fibrosis: a regression approach to determining the impact of recombinant human DNase. Pharmacotherapy. 1999;19(10):1159–66.
Huot L, Durieu I, Bourdy S, Ganne C, Bellon G, Colin C, et al. Evolution of costs of care for cystic fibrosis patients after clinical guidelines implementation in a French network. J Cyst Fibros. 2008;7(5):403–8.
Vertex Pharmaceuticals Inc. Republic of Ireland approves funding for KALYDECO™ (ivacaftor), the first medicine to treat the underlying cause of cystic fibrosis, for people with a specific genetic mutation (G551D). 2013. http://investors.vrtx.com/releasedetail.cfm?releaseid=737420. Accessed 5 July 2017.
Vertex Pharmaceuticals Inc. Vertex Announces long-term reimbursement agreement with the Republic of Ireland for ORKAMBI® (lumacaftor/ivacaftor), KALYDECO® (ivacaftor) and Future Cystic Fibrosis Medicines. 2017. http://investors.vrtx.com/releasedetail.cfm?releaseid=1028700. Accessed 5 July 2017.
National Centre for Pharmacoeconomics. Cost-effectiveness of Ivacaftor (Kalydeco™) for the treatment of cystic fibrosis in patients age 6 years and older who have the G551D mutation. 2013. http://www.ncpe.ie/wp-content/uploads/2012/08/Ivacaftor-Summary.pdf. Accessed 5 July 2017.
National Centre for Pharmacoeconomics. Cost-effectiveness of Lumacaftor/Ivacaftor (Orkambi) for cystic fibrosis in patients aged 12 years and older who are homozygous for the F508del mutation in the CFTR gene. 2016. http://www.ncpe.ie/wp-content/uploads/2015/12/Website-summary-orkambi.pdf. Accessed 5 July 2017.
De Boeck K, Zolin A, Cuppens H, Olesen HV, Viviani L. The relative frequency of CFTR mutation classes in European patients with cystic fibrosis. J Cyst Fibros. 2014;13(4):403–9.
Baumann U, Stocklossa C, Greiner W, von der Schulenburg JM, von der Hardt H. Cost of care and clinical condition in paediatric cystic fibrosis patients. J Cyst Fibros. 2003;2(2):84–90.
Eidt-Koch D, Wagner TO, Mittendorf T, Graf von der Schulenburg JM. Outpatient medication costs of patients with cystic fibrosis in Germany. Appl Health Econ Health Policy. 2010;8(2):111–8.
Goss CH, Burns JL. Exacerbations in cystic fibrosis. 1: epidemiology and pathogenesis. Thorax. 2007;62(4):360–7.
Jackson AD, Harrington M, Zhou S, Daly L, Kelleher C, Fitzpatrick P, et al. Delayed cystic fibrosis presentation in children in the absence of newborn screening. Ir Med J. 2010;103(4):113–6.
National Casemix Pogramme of the Health Services Executive. Ready reckoner of acute hospital inpatient and daycase activity and costs (summarised by DRG) relating to 2011 costs and activity. Dublin: National Casemix Programme; 2014.
O’Brien C, Fogarty E, Walsh C, Dempsey O, Barry M, Kennedy MJ, et al. The cost of the inpatient management of febrile neutropenia in cancer patients—a micro-costing study in the Irish healthcare setting. Eur J Cancer Care. 2014;24(1):125–32.
Kaplan RS. Improving value with TDABC. Healthc Financ Manag. 2014;68(6):76–83.
Kaplan RS, Haas DA. How not to cut health care costs. Harv Bus Rev. 2014;92(11):116–22.
Kaplan RS, Porter ME. The big idea: how to solve the cost crisis in health care. Harv Bus Rev. 2011;89:46–52.
Kaplan RS, Porter ME. Managing healthcare costs and value. Strategic Finance. 2017;98(7):24–33.
Kaplan RS, Witkowski ML. Better accounting transforms health care delivery. Account Horiz. 2014;28(2):365–83.
Kaplan RS, Witkowski ML, Abbott M, Guzman A, Higgins L, Meara J, et al. Using time-driven activity-based costing to identify value-improvement opportunities in healthcare. J Healthc Manag. 2014;59(6):399–413.
Porter ME, Lee TH. The strategy that will fix health care. Harv Bus Rev. 2013;91(10):50–70.
Angelis A, Tordrup D, Kanavos P. Socio-economic burden of rare diseases: a systematic review of cost of illness evidence. Health Policy. 2015;119(7):964–79.
Acknowledgements
We thank Dr. Abhijeet Bhanegaonkar and Ellison Suthoff at Vertex Pharmaceuticals Incorporated for their editorial support during the preparation of this manuscript. The authors wish to thank the CF patients, their families and caregivers who have registered with the CFRI, the CF care teams and hospital management teams for releasing HIPE data, the National Centre for Pharmacoeconomics, the Health Service Executive’s Health Intelligence Unit and the Healthcare Pricing Office. We also wish to thank the journal’s reviewers for their thoughtful comments, which have substantially improved the quality and content of the work.
Author information
Authors and Affiliations
Contributions
A.D.J., A.L.J. and F.C. had complete access to the anonymised study dataset. A.D.J. and G.F. designed and conducted the study. A.D.J. and F.C. conducted a review of the literature and analysed the data with statistical support provided by A.L.J. G.D. provided support with the costing methodology. M.H. and S.Z. were responsible for CFRI patient recruitment and data collection. A.D.J. prepared the manuscript draft with important data interpretation and intellectual input from G.D., E.M. and C.G. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Funding
Funding support for this study was provided by Vertex Pharmaceuticals Incorporated.
Conflict of interest
A. D. Jackson, A. L. Jackson, G. Fletcher, G. Doyle, M. Harrington, S. Zhou, F. Cullinane, C. Gallagher and E. McKone declare that they have no conflicts of interest.
Data availability statement
All the relevant data is presented within the main manuscript and supplementary files. The CFRI dataset is not a freely accessible dataset. Requests for anonymous data/information from the CFRI are considered, and are subjected to a data application process. Data/information requests are considered by CFRI’s Research Committee whose decision whether to provide data or information is final.
Additional information
E. McKone: on behalf of the Cystic Fibrosis Registry of Ireland (CFRI) Executive Committee.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jackson, A.D., Jackson, A.L., Fletcher, G. et al. Estimating Direct Cost of Cystic Fibrosis Care Using Irish Registry Healthcare Resource Utilisation Data, 2008–2012. PharmacoEconomics 35, 1087–1101 (2017). https://doi.org/10.1007/s40273-017-0530-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-017-0530-4