Abstract
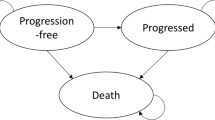
As part of the National Institute for Health and Care Excellence (NICE) single technology appraisal process, the manufacturer of crizotinib submitted evidence on the clinical and cost effectiveness of crizotinib in untreated anaplastic lymphoma kinase-positive (ALK-positive) non-small-cell lung cancer (NSCLC). Crizotinib has previously been assessed by NICE for patients with previously treated ALK-positive NSCLC (TA 296). It was not approved in this previous appraisal, but had been made available through the cancer drugs fund. As part of this new appraisal, the company included a price discount patient access scheme (PAS). The Centre for Reviews and Dissemination and Centre for Health Economics Technology Appraisal Group at the University of York was commissioned to act as the independent Evidence Review Group (ERG). This article provides a description of the company’s submission and the ERG’s review and summarises the resulting NICE guidance issued in August 2016. The main clinical-effectiveness data were derived from a multicentre randomised controlled trial—PROFILE 1014—that compared crizotinib with pemetrexed chemotherapy in combination with carboplatin or cisplatin in patients with untreated non-squamous ALK-positive NSCLC. In the trial, crizotinib demonstrated improvements in progression-free survival (PFS) and overall survival (OS). The company’s economic model was a three-state ‘area under the curve’ Markov model. The base-case incremental cost-effectiveness ratio (ICER) was estimated to be greater than £50,000 per quality-adjusted life-year (QALY) gained (excluding the PAS discount). The ERG assessment of the evidence submitted by the company raised a number of concerns. In terms of the clinical evidence, the OS benefit was highly uncertain due to the cross-over permitted in the trial and the immaturity of the data; only 26% of events had occurred by the data cut-off point. In the economic modelling, the most significant concerns related to the analysis of OS and assumptions made regarding the duration of therapy. The ERG exploratory re-analysis of the OS data relaxed the assumption of proportional hazards made in the company submission, which demonstrated significant uncertainty regarding the OS gains from crizotinib. The ERG reconfigured the economic model so that duration of therapy was based on the area under the curve analysis of the PROFILE 1014 trial, dramatically increasing the cost associated with implementing crizotinib and consequently, substantially increasing the ICER. At the first appraisal meeting, the NICE Appraisal Committee concluded that crizotinib, while clinically effective, was not sufficiently cost effective for use in the UK NHS. Following the consultation, the company offered a revised PAS and conducted extensive re-analysis, resulting in a revised base-case ICER of £47,291 per QALY gained. The NICE Appraisal Committee concluded that crizotinib was likely to be a cost-effective use of NHS resources, despite the uncertainty that persisted around a number of factors, namely the long-term survival benefit of crizotinib. Crizotinib was therefore recommended as an option for untreated ALK-positive advanced NSCLC in adults.

Similar content being viewed by others
References
National Institute for Health and Care Excellence. Crizotinib for previously treated non-small-cell lung cancer associated with an anaplastic lymphoma kinase fusion gene. Technology appraisal guidance [TA296]. London: NICE; 2013. https://www.nice.org.uk/guidance/ta296. Accessed 11 Nov 2016.
National Institute for Health and Care Excellence. Crizotinib for untreated anaplastic lymphoma kinase-positive advanced non-small-cell lung cancer. Technology appraisal guidance [TA406]. London: NICE; 2016. https://www.nice.org.uk/guidance/ta406. Accessed 11 Nov 2016.
Royal College of Physicians. National Lung Cancer Audit (NCLA) annual report 2015 for the audit period 2014. http://www.hqip.org.uk/resources/lung-cancer-audit-annual-report-2015/. Accessed 11 Nov 2016.
American Cancer Society. Non-small cell lung cancer signs and symptoms. http://www.cancer.org/cancer/lungcancer-non-smallcell/detailedguide/non-small-cell-lung-cancer-signs-symptoms. Accessed 11 Nov 2016.
Cancer Research UK. Lung Cancer Incidence Statistics: morphology. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/lung-cancer/incidence#heading-Four. Accessed 11th Nov 2016.
Clinical Lung Cancer Genome Project (CLCGP), Network Genomic Medicine (NGM). A genomics-based classification of human lung tumors. Sci Transl Med. 2013;5(209):209.
Bang YJ. The potential for crizotinib in non-small cell lung cancer: a perspective review. Ther Adv Med Oncol. 2011;3(6):279–91.
Hirsh V, Cadranel J, Cong XJ, Fairclough D, Finnern HW, Lorence RM, et al. Symptom and quality of life benefit of afatinib in advanced non-small-cell lung cancer patients previously treated with erlotinib or gefitinib: Results of a randomized phase IIb/III trial (LUX-lung 1). J Thorac Oncol. 2013;8(2):229–37.
Cancer Research UK. Lung Cancer Statistics: incidence. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/lung-cancer#heading-Zero. Accessed 11 Nov 2016.
Cancer Research UK. Lung Cancer: survival. http://www.cancerresearchuk.org/about-cancer/type/lung-cancer/treatment/statistics-and-outlook-for-lung-cancer. Accessed 11 Nov 2016.
National Institute for Health and Care Excellence. Lung cancer: diagnosis and management. Treatment. Clinical guideline [CG121]. London: NICE; 2011. https://www.nice.org.uk/guidance/cg121/chapter/1-Guidance#treatment. Accessed 11 Nov 2016.
Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26(21):3543–51.
Shaw AT, Yeap BY, Solomon BJ, Riely GJ, Gainor J, Engelman JA, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol. 2011;12(11):1004–12.
Grønberg BH, Bremnes RM, Fløtten O, et al. Phase III study by the Norwegian lung cancer study group: pemetrexed plus carboplatin compared with gemcitabine plus carboplatin as first-line chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2009;27(19):3217–22.
Solomon BJ, Mok T, Kim D-W, Wu Y-L, Nakagawa K, Mekhail T, et al. First-Line Crizotinib versus Chemotherapy in ALK-Positive Lung Cancer. N Engl J Med. 2014;371(23):2167–77.
Camidge DR, Bang YJ, Kwak EL, Iafrate AJ, Varella-Garcia M, Fox SB, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13(10):1011–9.
Davis KL, Kaye JA, Iyer S. Response Rate and Outcomes in Crizotinib Treated Advanced ALK-positive NSCLC Patients. Presented at the 16th World Conference on Lung Cancer; 6–9 Sept 2015, Denver.
European Medicines Agency. Xalkori. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002489/human_med_001592.jsp&mid=WC0b01ac058001d124. Accessed 11 Nov 2016.
Latimer N. NICE DSU technical support document 14: survival analysis for economic evaluations alongside clinical trials - extrapolation with patient-level data. 2011 June (last updated March 2013). http://www.nicedsu.org.uk/NICE%20DSU%20TSD%20Survival%20analysis.updated%20March%202013.v2.pdf. Accessed 11 Nov 2016.
National Institute for Health and Care Excellence. Nintedanib for previously treated locally advanced, metastatic, or locally recurrent non-small-cell lung cancer. Technology appraisal guidance [TA347]. London: NICE; 2015. http://www.nice.org.uk/guidance/ta347. Accessed 11 Nov 2016.
European Medicines Agency. Alimta: Annex I: Summary of Product Characteristics. Fegersheim: Lilly France. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000564/WC500025611.pdf. Accessed 11 Nov 2016.
Cancer Research UK. Molecular diagnostic provision in England: for Targeted cancer medicines (solid tumour) in the NHS. 2015. https://www.cancerresearchuk.org/sites/default/files/policy_august2015_mdx_final_1.pdf. Accessed 11 Nov 2016.
National Institute for Health and Care Excellence. Ceritinib for previously treated anaplastic lymphoma kinase positive non-small-cell lung cancer. Technology appraisal guidance [TA395]. London: NICE; 2016. https://www.nice.org.uk/Guidance/TA395. Accessed 11 Nov 2016.
Acknowledgements
The authors thank Dr Katy Clarke, Consultant Clinical Oncologist at St. James’ University Hospital Leeds, and Dr Neal Navani, Consultant in Thoracic Medicine at UCLG & MRC Clinical Trials Unit and Clinical Lead for Lung Cancer and Bronchoscopy at UCLH, for clinical advice throughout the project.
Author contributions
Robert Hodgson, Mousumi Biswas, Philip Morgan, Teumzghi Mebrahtu Melissa Harden and Nerys Woolacott all formed the part of the ERG that produced the ERG report described in this paper. Philip Morgan and Robert Hodgson wrote the first draft of the manuscript. All authors commented on the manuscript and approved the final version. This summary has not been externally reviewed by PharmacoEconomics.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This project was funded by the National Institute for Health Research (NIHR) Health Technology Assessment Programme (project number 15/121/10) and will be published as part of a compendium of ERG articles in Health Technology Assessment. See the HTA programme website (http://www.hta.ac.uk) for further project information. This summary of the ERG report was compiled after the Appraisal Committee’s review and incorporates additional information and comment from the authors on the STA process and iterations of the NICE guidance not covered by the HTA report. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of NICE or the Department of Health. This work is Crown copyright (UK).
Conflict of interest
Philip Morgan, Nerys Woolacott, Mousumi Biswas, Teumzghi Mebrahtu, Melissa Harden and Robert Hodgson have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Morgan, P., Woolacott, N., Biswas, M. et al. Crizotinib for Untreated Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: An Evidence Review Group Perspective of a NICE Single Technology Appraisal. PharmacoEconomics 35, 909–919 (2017). https://doi.org/10.1007/s40273-017-0497-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-017-0497-1