Abstract
Background
Protease inhibitor (PI) monotherapy can maintain virological suppression in the majority of patients once it has been established on triple therapy and may also have the potential for substantial cost savings arising from the use of fewer drugs. However, the cost effectiveness of PI monotherapy has yet to be demonstrated.
Objectives
In this study we examine the cost effectiveness of PI monotherapy with prompt return to combination therapy in the event of viral load rebound compared with ongoing triple therapy (OT) in patients with suppressed viral load on combination antiretroviral therapy (ART) in the UK.
Methods
The analysis used data from the PIVOT trial in which HIV-positive adults with suppressed viral load for ≥24 weeks on combination ART were randomised to maintain OT or to a strategy of PI monotherapy with prompt return to combination therapy if viral load rebounded. A cost-effectiveness analysis including long-term modelling was conducted. Main outcomes included UK National Health Service (NHS) costs and quality-adjusted life-years (QALYs) with comparative results presented as incremental cost-effectiveness ratios.
Results
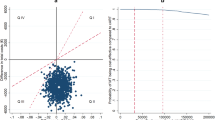
PI monotherapy was cost saving as a result of large savings in ART drug costs while being no less effective in terms of QALYs in the within-trial analysis and marginally less effective with lifetime modelling. In the base-case analysis over 3 years, the incremental total cost per patient was −£6424.11 (95 % confidence interval −7418.84 to −5429.38) and incremental QALYs were 0.0051 (95 % CI −0.0479 to 0.0582), resulting in PI monotherapy ‘dominating’ OT. Multiple scenario analyses found that PI monotherapy was cost saving with no marked differences in QALYs. Modelling of lifetime costs and QALYs showed that PI monotherapy was associated with significant cost savings and was marginally less effective; PI monotherapy was cost effective at accepted cost-effectiveness thresholds in all but one scenario analysis.
Conclusions
Under most assumptions, PI monotherapy appears to be a cost-effective treatment strategy compared with OT for HIV-infected patients who have achieved sustained virological suppression.
Similar content being viewed by others
References
Williams I, Churchill D, Anderson J, Boffito M, Bower M, Cairns G, et al. British HIV Association guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2012. HIV Med. 2012;13(Suppl. 2):1–85.
Thompson MA, Aberg JA, Hoy JF, Benson C, Gu HF, Hammer SM, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA Panel. JAMA. 2014;308(4):387–402.
Mathis S, Khanlari B, Pulido F, Schechter M, Negredo E, Nelson M, et al. Effectiveness of protease inhibitor monotherapy versus combination antiretroviral maintenance therapy: a meta-analysis. PLoS One. 2011;6(7):e22003.
Restelli U, Croce D, Porazzi E, Scolari F, Bonfanti M, Galli M, et al. Health technology assessment in the HIV setting: the case of monotherapy. New Microbiol. 2014;37(3):247–61.
Gazzard B, Hill A, Anceau A. Cost-efficacy analysis of the MONET trial using UK antiretroviral drug prices. Appl Health Econ Health Policy. 2011;9(4):217–23.
Paton NI, Stöhr W, Arenas-Pinto A, Fisher M, Williams I, Johnson M, et al. Protease inhibitor monotherapy for long-term management of HIV infection: a randomised, controlled, open-label, non-inferiority trial. Lancet HIV. 2015;2(10):e417–26.
Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. 3rd ed. New York: Oxford University Press; 2005.
National Institute for Health and Care Excellence (NICE). Guide to the methods of technology appraisal. London: NICE; 2013.
EuroQol Group. What is EQ-5D. 2013. http://www.euroqol.org/faqs/eq-5d-3l.html. Accessed 11 Jan 2016.
Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35(11):1095–108.
British Medical Association and Royal Pharmaceutical Society of Great Britain. British National Formulary. London: Pharmaceutical Press; 2013.
Department of Health Commercial Medicines Unit. Electronic Market Information Tool (eMit). Canterbury: Personal Social Services Research Unit; 2013.
Curtis L. Unit costs of health and social care 2012. Kent: Personal Social Services Research Unit, The University of Kent; 2012.
National Health Service (NHS). NHS reference costs: financial year 2011 to 2012. 2012. http://cmu.dh.gov.uk/electronic-market-information-tool-emit/. Accessed 6 June 2014.
Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess. 2015;19(14):1–503, v–vi.
Horton NJ, Kleinman KP. Much ado about nothing: a comparison of missing data methods and software to fit incomplete data regression models. Am Stat. 2007;61(1):79–90.
Royston P. Multiple imputation of missing values. Stata J. 2004;4(3):227–41.
Lloyd JEV, Obradovic J, Carpiano RM, Frosso M-S. Multiple imputation of missing multilevel, longitudinal data: a case when practical considerations trump best practices? J Mod Appl Stat Methods. 2013;12(1):261–75.
Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev Sci. 2007;8(3):206–13.
Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. Pharmacoeconomics. 2014;32(12):1157–70.
Barber J, Thompson S. Multiple regression of cost data: use of generalised linear models. J Health Serv Res Policy. 2004;9(4):197–204.
Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ. 2005;14(5):487–96.
Briggs A, Claxton K, Sculpher M. Decision modelling for health economic evaluation. 1st ed. New York: Oxford University Press; 2006.
Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ. 2001;10(8):779–87.
May MT, Gompels M, Delpech V, Porter K, Orkin C, Kegg S, et al. Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS. 2014;28(8):1193–202.
May M, Gompels M, Delpech V, Porter K, Post F, Johnson M, et al. Impact of late diagnosis and treatment on life expectancy in people with HIV-1: UK Collaborative HIV Cohort (UK CHIC) study. BMJ. 2011;343:1–11.
Mills J, Knipe E. Historic and projected mortality data from the period and cohort life tables, 2012-based, UK, 1981–2062. 2013. http://www.ons.gov.uk/ons/dcp171778_345078.pdf. Accessed 11 Jan 2016.
Sullivan PW, Slejko JF, Sculpher MJ, Ghushchyan V. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Making. 2011;31(6):800–4.
Feeney ER, Chung RT, Yazdanpanah Y. Current guidelines and prioritizing treatment of hepatitis C virus in HIV-infected patients. Curr Opin HIV AIDS. 2015;10(5):323–9. http://journals.lww.com/co-hivandaids/Abstract/2015/09000/Current_guidelines_and_prioritizing_treatment_of.6.aspx. Accessed 24 Aug 2015.
The INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. http://www.nejm.org/doi/full/10.1056/NEJMoa1506816. Accessed 24 Aug 2015.
Yin Z, Brown A, Hughes G, Nardone A, Gill ON, Delpech V. HIV in the United Kingdom: 2014 report. London; 2014. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/401662/2014_PHE_HIV_annual_report_draft_Final_07-01-2015.pdf. Accessed 24 Aug 2015.
O’Brien BJO, Gertsen K, Willan AR, Faulkner LA. Is there a kink in consumers’ threshold value for cost-effectiveness in health care? Health Econ. 2002;11:175–80.
Arribas JR, Horban A, Gerstoft J, Fätkenheuer G, Nelson M, Clumeck N, et al. The MONET trial: darunavir/ritonavir with or without nucleoside analogues, for patients with HIV RNA below 50 copies/ml. AIDS. 2010;24(2):223–30.
Puls R, Amin J, Losso M, Phanuphak P, Nwizu C, Orrell C, et al. Efficacy of 400 mg efavirenz versus standard 600 mg dose in HIV-infected, antiretroviral-naive adults (ENCORE1): a randomised, double-blind, placebo-controlled, non-inferiority trial. Lancet (London, England). 2014;383(9927):1474–82. http://ww.ncbi.nlm.nih.gov/pubmed/24522178. Accessed 24 Aug 2015.
Miners A, Phillips A, Kreif N, Rodger A, Speakman A, Fisher M, et al. Health-related quality-of-life of people with HIV in the era of combination antiretroviral treatment: a cross-sectional comparison with the general population. Lancet HIV. 2014;1(1):e32–40. http://ww.sciencedirect.com/science/article/pii/2352301814700189. Accessed 6 Jan 2016.
Clayson DJ, Wild DJ, Quarterman P, Duprat-Lomon I, Kubin M, Coons SJ. A comparative review of health-related quality-of-life measures for use in HIV/AIDS clinical trials. Pharmacoeconomics. 2006;24(8):751–65. http://link.springer.com/10.2165/00019053-200624080-00003. Accessed 6 Jan 2016.
Acknowledgments
We thank all of the patients and staff from all of the centres participating in the PIVOT trial, and the UK Community Advisory Board and African Eye for community support.
Author contributions
DD, NP and MS designed the study. WS, AA-P, DD and NP contributed to the coordination and oversight of the study. LO, SW and MS conducted the health economics analysis. All authors participated in data interpretation. The manuscript was drafted by LO, SW and MS and all authors provided input into the report and approved the final version of the manuscript.
The PIVOT Trial Team
Participating UK Sites: Elton John Centre, Brighton: Martin Fisher, Amanda Clarke, Wendy Hadley, David Stacey. Royal Free Hospital, London: Margaret Johnson, Pat Byrne. Mortimer Market Centre, London: Ian Williams, Nahum De Esteban, Pierre Pellegrino, Lewis Haddow, Alejandro Arenas-Pinto. Barts and The London Hospital: Chloe Orkin, James Hand, Carl De Souza, Lisa Murthen, Andrew Crawford-Jones. Royal Berkshire Hospital, Reading: Fabian Chen, Ruth Wilson, Elizabeth Green, John Masterson. Manchester Royal Infirmary, Manchester: Vincent Lee, Kamlesh Patel, Rebecca Howe. St Mary’s Hospital, London: Alan Winston, Scott Mullaney. Southmead Hospital, Bristol: Mark Gompels, Louise Jennings. Royal Liverpool University Hospital, Liverpool: Nicholas Beeching, Rebecca Tamaklo. Guys and St Thomas’ Hospital, London: Julie Fox, Alistair Teague, Isabelle Jendrulek, Juan Manuel Tiraboschi. North Manchester General Hospital, Manchester: Ed Wilkins, Yvonne Clowes, Andrew Thompson. Central Middlesex Hospital, London: Gary Brook, Manoj Trivedi. Avenue House Clinic, Eastbourne: Kazeem Aderogba, Martin Jones. Gloucester Royal Hospital, Gloucester: Andrew DeBurgh-Thomas, Liz Jones. Homerton University Hospital, London: Iain Reeves, Sifiso Mguni. James Cook University Hospital, Middlesbrough: David Chadwick, Pauline Spence, Nellie Nkhoma. Derriford Hospital, Plymouth: Zoe Warwick, Suzanne Price, Sally Read. Royal Bournemouth Hospital, Bournemouth: Elbushra Herieka, James Walker, Ruth Woodward. Southend University Hospital, Westcliff-on-Sea: John Day, Laura Hilton. St Mary’s Hospital, Portsmouth: Veerakathy Harinda, Helen Blackman. St George’s Hospital, London: Phillip Hay, Wendy Mejewska, Olanike Okolo. Royal Victoria Infirmary, Newcastle: Edmund Ong, Karen Martin, Lee Munro. Royal Hallamshire Hospital, Sheffield: David Dockrell, Lynne Smart. North Middlesex University Hospital, London: Jonathan Ainsworth, Anele Waters. Queen Elizabeth Hospital, Woolwich: Stephen Kegg, Sara McNamara. Birmingham Heartlands Hospital, Birmingham: Steve Taylor, Gerry Gilleran. Chelsea and Westminster Hospital, London: Brian Gazzard, Jane Rowlands. University Hospital of Coventry, Coventry: Sris Allan, Rumun Sandhu. Ealing Hospital, London: Nigel O’Farrell, Sheena Quaid. Harrogate District Hospital, Harrogate: Fabiola Martin, Caroline Bennett. Northwick Park Hospital, Harrow: Moses Kapembwa. St James’ Hospital, Leeds: Jane Minton, James Calderwood. King’s College Hospital, London: Frank Post, Lucy Campbell, Emily Wandolo. Leicester Royal Infirmary, Leicester: Adrian Palfreeman, Linda Mashonganyika. Luton and Dunstable Hospital, Luton: Thambiah Balachandran, Memory Kakowa. Newham University Hospital, London: Rebecca O’Connell, Cheryl Tanawa. Edith Cavell Hospital, Peterborough: Sinna Jebakumar, Lesley Hagger. Royal Victoria Hospital, Belfast: Say Quah, Sinead McKernan. York Teaching Hospital, York: Charles Lacey, Sarah Douglas, Sarah Russell-Sharpe, Christine Brewer. Western General Hospital, Edinburgh: Clifford Leen, Sheila Morris. Barking Hospital, London: Sharmin Obeyesekera, Shirley Williams. Norfolk and Norwich University Hospital, Norwich: Nelson David. Worcester Royal Hospital, Worchester: Mark Roberts, Julie Wollaston.
MRC Clinical Trials Unit at UCL: Nicholas Paton, Wolfgang Stöhr, Alejandro Arenas-Pinto, Karen Scott, David Dunn, Emma Beaumont, Sue Fleck, Mark Hall, Susie Hennings, Ischa Kummeling, Sara Martins, Ellen Owen-Powell, Karen Sanders, Fionna van Hooff, Livia Vivas, Ellen White.
Independent Event Reviewer: Brian Angus.
Trial Steering Committee: Andrew Freedman (Chair), Ben Cromerty, Danielle Mercey, Sarah Fidler, Estee Torok, Abdel Babiker, Brian Gazzard, Chloe Orkin, Nicholas Paton.
Data Monitoring Committee: Tim Peto (Chair), David Lalloo, Andrew Phillips and Robert James.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Ethics committee
The protocol was approved by the Cambridgeshire 4 Research Ethics Committee and the Medicines and Healthcare Products Regulatory Agency and has been performed in accordance with the ethical standards of the Declaration of Helsinki.
All participants provided written informed consent.
Conflicts of interest
LO reports funding from the North Denmark Region and Aalborg University and has worked as a paid consultant for Novartis Healthcare; SW reports grants from the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme during the conduct of the study; WS reports grants from NIHR HTA programme during the conduct of the study; AA-P reports non-financial support from UK Clinical Research Network during the conduct of the study and grants from Janssen and ViiV outside the submitted work; DD reports grants from the NIHR HTA programme during the conduct of the study; NP reports grants from NIHR HTA programme during the conduct of the study, and grants, personal fees and non-financial support from AbbVie, personal fees and non-financial support from Merck, grants, personal fees and non-financial support from Janssen, personal fees and non-financial support from Roche, grants and non-financial support from GSK, and non-financial support from Gilead outside the submitted work; MS reports grants from NIHR HTA programme during the conduct of the study and consultancy fees from Gilead.
Source of support
PIVOT was funded by the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme (project number 06/403/90). The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the HTA programme, NIHR, the National Health Service (NHS) or the Department of Health.
Additional information
Members of the PIVOT Trial Team are listed in the Acknowledgments.
Rights and permissions
About this article
Cite this article
Oddershede, L., Walker, S., Stöhr, W. et al. Cost Effectiveness of Protease Inhibitor Monotherapy Versus Standard Triple Therapy in the Long-Term Management of HIV Patients: Analysis Using Evidence from the PIVOT Trial. PharmacoEconomics 34, 795–804 (2016). https://doi.org/10.1007/s40273-016-0396-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-016-0396-x