Abstract
Background
Biosimilars are medicinal products that are similar to a biopharmaceutical that has already been authorised. As biopharmaceuticals are expected to dominate the best-selling pharmaceuticals worldwide by 2016, the emergence of biosimilars imposes an important challenge for governments. At this moment, the uptake of biosimilars in Belgium is limited, with market shares close to 0 %.
Objective
This study aimed to identify the barriers that impede the uptake of biosimilars in Belgium.
Methods
Semi-structured interviews were conducted to investigate in depth the barriers to the uptake of biosimilars in Belgium. Respondents were selected through selective sampling so that all different stakeholders were represented (authorities, physicians, pharmacists, patients, academics and industry). Respondents were contacted by e-mail and letter with a request for participation. A thematic framework was used to analyze the data.
Results
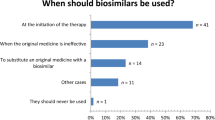
Three main barriers to the uptake of biosimilars in the Belgian market were identified: a lack of confidence towards biosimilars by some stakeholders; uncertainty about the interchangeability and substitution of biosimilars; and a hospital financing system that discourages the use of them. Providing all stakeholders with objective information on the concept of biosimilars, reforming the financing of hospitals, developing and implementing prescription quota in hospitals, setting up patient registries for biosimilars and speeding up the pricing and reimbursement process of biosimilars are suggested solutions to increase the uptake of biosimilars in Belgium.
Conclusions
To fully capture the potential savings of biosimilars, governments should take measures to increase their uptake. The Belgian government, and also the manufacturers of biosimilars, should take measures to reduce the uncertainties related to biosimilars and raise confidence among prescribers. In addition, the financing of hospitals should be reformed and incentives should be developed to stimulate physicians to prescribe biosimilars.
Similar content being viewed by others
References
GaBi Online: Generics and Biosimilars Initiative. Biologicals sales have almost doubled since 2006. Available from: http://www.gabionline.net/Biosimilars/General/Biologicals-sales-have-almost-doubled-since-2006.
PipelineReview.com. Blockbuster biologics 2012. R&D Pipeline News, Special Edition 1/2013. Available from: http://www.pipelinereview.com/index.php/2013050850905/FREE-Reports/Blockbuster-Biologics-2012.html.
Windisch J. The science in biosimilars. Emergence of Biosimilar Medicines Symposium, Belgian Federal Parliament, 22 November 2012. Available from: http://www.sympobiosimilars.be/fr/docs/Symposium_Biosimilars_22112012_Presentation_3.pdf
Weise M, Bielsky M-C, De Smet K, Ehmann F, Ekman N, Narayanan G, et al. Biosimilars—why terminology matters. Nat Biotechnol. 2012;29(8):690–3.
Declerck P, Simoens S. A European perspective on the market accessibility of biosimilars. Biosimilars. 2012;2:33–40.
European Generic Medicines Association. Biosimilars handbook. 2nd ed. EGA; 2011.
Schiestl M, Stangler T, Torella C, Cepeljnik T, Toll H, Grau R. Acceptable changes in quality attributes of glycosylated biopharmaceuticals. Nat Biotechnol. 2011;29(4):310–2.
European Commission. Directive 2001/83/EC Art. 10(4) and Part II of the Annex I of Directive 2001/83/EC, as amended. Eudralex. 2013; 1.
European Commission. What you need to know about Biosimilar Medicinal Products? Brussels: European Commission; 2013.
Elsevier Clinical Decision Support. Biosimilars—US and international update. Amsterdam: Elsevier; 2012.
European Medicines Agency. EMA procedural advice for users of the Centralised Procedure for Similar Biological Medicinal Products applications. EMA/940451/2011. London: European Medicines Agency; 2012.
GBI Research. Biosimilars approval pathways in the US and Europe—development and approval of biosimilar mABs may face tough regulatory environment. GBI Research, editor; 2011.
Biosimilars—an update. Advisory Committee for Pharmaceutical Science and Clinical Pharmacology, Food and Drug Administration; 2012.
Gaffney A. FDA releases fourth biosimilar guidance outlining new types of meetings. Regulatory Focus. 1 Apr 2013. Available from: https://www.raps.org/focus-online/news/news-article-view/article/3106/fda-releases-fourth-biosimilar-guidance-outlining-new-types-of-meetings.aspx.
Simoens S, Verbeken G, Huys I. Biosimilars and market access: a question of comparability and costs? Target Oncol. 2012;7:227–31.
Dunne S, Shannon B, Dunne C, Cullen W. A review of the differences and similarities between generic drugs and their originator counterparts, including economic benefits associated with usage of generic medicines, using Ireland as a case study. BMC Pharmacol Toxicol 2013;14(1):1–19.
Bourgoin AF, Nuskey B. An outlook on US biosimilar competition. New York: Thomson Reuters; 2013.
Grabowski H, Guha R, Salgado M. Biosimilar competition: lessons from Europe. Nat Rev Drug Discov. 2014;13:99–100.
Rovira J, Espin J, Garcia L, Olry de Labry A. The impact of biosimilars’ entry in the EU market. Andalusia: Andalusian School of Public Health; 2011.
Haustein R, de Millas C, Höer A, Häussler B. Saving money in the European healthcare systems with biosimilars. GaBi J. 2012;3–4:120–6.
Befrits G. The case for biosimilars: a payer’s perspective. GaBi J. 2013;2(1):21.
GaBi. US$67 billion worth of biosimilar patents expiring before 2020. GaBi Online: Generics and Biosimilars Initiative; 2012.
European Medicines Agency. European public assessment reports. 2013. Available from: http://www.ema.europa.eu.
Lepage-Nefkens I, Gerkens S, Vinck I, Piérart J, Hulstaert F, Farfan-Portet M-I. Barriers and opportunities for the uptake of biosimilar medicines in Belgium. 199. Brussels: KCE Health Services Research; 2013.
European Medicines Agency. European Medicines Agency recommends approval of first two monoclonal-antibody biosimilars. 2013. Available from: http://www.ema.europa.eu.
Blank T, Netzer T, Hildebrandt W, Vogt-Eisele A, Kaszkin-Bettag M. Safety and toxicity of biosimilars: EU versus US regulation. GaBi J. 2013;2(3):144–50.
European Commission. Reimbursement status of authorised biosimilars in MS and EFTA countries. Brussels: European Commission; 2013.
IMS. Biosimilar accessible market: size and biosimilar penetration. London; ims | Intelligence Applied; 2012.
Sheppard A. Generic medicines, what can the future hold? London: SMi Generics and Patent Strategies; 14 May 2013.
Greenland P. Emergence of biosimilar medicines: the biosimilar company point of view. Belgium Federal Parliament Brussels; 22 November 2012.
Di Biase S. Biosimilars in the European Market: why and how this is the new frontier? Warsaw: IMS; 2013.
Moors E. Challenges for the adoption of future biosimilars. Eur J Hosp Pharmacy Pract. 2007;13(5):57–8.
Pope C, Mays N. Qualitative research in health care. 3rd ed. Oxford: Blackwell Publishing; 2006.
QSR Nvivo 9 for Windows. QRS International Pty Ltd; 2006.
Crommelin D, Vlieger J, Weinstein V, Mühlebach S, Shah VP, Schellekens H. Different pharmaceutical products need similar terminology. AAPS J. 2014;16(1):11–4.
The emergence of biosimilars: which opportunities for patients and the Health Insurance? Belgian Federal Parliament; 22 November 2012.
European Generic Medicines Association. EC Project on market access and uptake of biosimilars: survey to members states and EEA countries on biosimilars. Brussels: EGA; 2012.
Vulto A. Biosimilars, information gap and barriers to substitution. 9th EGA Symposium on Biosimilar Medicines. London: 2011.
Godman B. Health authority perspective on biosimilars. GaBi J. 2013;2(1):10–1.
Ebbers H, Muenzberg M, Schellekens H. The safety of switching between therapeutic proteins. Expert Opin Biol Ther. 2012;12(11):1473–85.
Simoens S. Health economics of market access for biopharmaceuticals and biosimilars. J Med Econ. 2009;12(3):211–8.
Coudron A, Lambrechts J, Landrieux T, Max J, Rogiers A. Research on market access for biosimilars: detecting obstacles and possible solutions. Leuven: KU Leuven; 2013.
Bocquet F, Paubel P, Fusier I, Cordonnier A-L, Le Pen C, Sinègre M. Biosimilar granulocyte colony-stimulating factor uptakes in the EU-5 markets: a descriptive analysis. Appl Health Econ Health Policy. Epub 1 Mar 2014. doi:10.1007/s40258-014-0087-8.
Derbyshire M. US state legislation on biosimilars substitution. GaBi J. 2013;2(3):155–6.
GaBi. US state biosimilar substitution bill becomes law. GaBi Online: Generics and Biosimilars Initiative; 2013.
GaBi. Fourth US state rejects law restricting biosimilar substitution. GaBi Online: Generics and Biosimilars Initiative; 2013.
GaBi. Biotech firms try to limit biosimilar substitution in US. GaBi Online: Generics and Biosimilars Initiative; 2013.
Generics and Biosimilars Initiative Online. 2013. Available from: http://www.gabionline.net.
Acknowledgments
The authors would like to thank A. Coudron, J. Lambrechts, T. Landrieux, J. Max and A. Rogiers for conducting the interviews and processing and analyzing the data. The authors would like to thank E. Picavet for her assistance during this research. No funding was received to conduct this research. S. Simoens holds the EGA Chair ‘European policy towards generic medicines’.Pieter Dylst, Arnold Vulto and Steven Simoens have no conflicts of interest that are directly relevant to the content of this manuscript.
Author contributions
The idea of the paper was developed by Steven Simoens and Pieter Dylst, based on a Master’s thesis of five pharmacy students. Pieter Dylst is the guarantor for the overall content. The manuscript was prepared by Pieter Dylst. Steven Simoens and Arnold Vulto contributed to this paper by reviewing the manuscript and adding suggestions to improve the paper. All authors revised the draft paper. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dylst, P., Vulto, A. & Simoens, S. Barriers to the Uptake of Biosimilars and Possible Solutions: A Belgian Case Study. PharmacoEconomics 32, 681–691 (2014). https://doi.org/10.1007/s40273-014-0163-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-014-0163-9