Abstract
Background
Over the last 15 years, a paradigm shift in oncology has led to the approval of dozens of targeted oral anti-cancer medications (OAMs), which have become the standard of care for certain cancers. While more convenient for patients than infused drugs, the possibility of non-adherence and the frequently high costs of targeted OAMs have proven controversial.
Objective
Our objective was to perform the first comprehensive review of cost-effectiveness analyses (CEAs) of targeted OAMs.
Methods
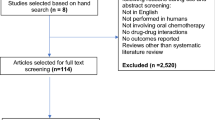
A literature search in PubMed, The Cochrane Library, and the Health Technology Assessment (HTA) reports published by the National Institute for Health Research HTA Programme in the UK was performed, covering articles published in the 5 years prior to 30 September 2013. Our inclusion criteria were peer-reviewed English-language full-text original research articles with a primary focus on CEA related to targeted OAMs. We categorized these articles by treatment setting (i.e. cancer site/type, line of therapy, and treatment and comparator) and synthesized information from the articles into summary tables.
Results
We identified 41 CEAs covering nine of the 18 targeted OAMs approved by the US FDA as of December 2012. These medications were studied in seven cancers, most often as second-line therapy for advanced-stage patients. In over half of treatment settings where a targeted OAM was compared with treatment that was not a targeted OAM, targeted OAMs were considered cost effective. Limitations in interpreting these findings include the risk of bias due to author conflicts of interest, cross-country variation, and difficulties in generalizing clinical trial evidence to community practice.
Conclusions
Several types of cost-effectiveness studies remain under-represented in the literature on targeted OAMs, including those for follow-on indications approved after the initial indication for a drug and for off-label indications, head-to-head comparisons of targeted OAMs with other targeted OAMs and targeted intravenous therapies, and studies that adopt a perspective other than the payer’s. Keeping up with the increasing number of approved targeted OAMs will also prove an important challenge for economic evaluation.

Similar content being viewed by others
References
Sledge GW Jr. What is targeted therapy? J Clin Oncol. 2005;23(8):1614–5.
Soria JC, Blay JY, Spano JP, Pivot X, Coscas Y, Khayat D. Added value of molecular targeted agents in oncology. Ann Oncol. 2011;22(8):1703–16.
Stuurman FE, Nuijen B, Beijnen JH, Schellens JH. Oral anticancer drugs: mechanisms of low bioavailability and strategies for improvement. Clin Pharmacokinet. 2013;52(6):399–414.
Geynisman DM, Wickersham KE. Adherence to targeted oral anticancer medications. Discov Med. 2013;15(83):231–41.
Weingart SN, Brown E, Bach PB, Eng K, Johnson SA, Kuzel TM, et al. NCCN Task Force Report: Oral chemotherapy. J Natl Compr Cancer Netw. 2008;6(Suppl 3):S1–14.
Zhu YX, Kortuem KM, Stewart AK. Molecular mechanism of action of immune-modulatory drugs thalidomide, lenalidomide and pomalidomide in multiple myeloma. Leuk Lymphoma. 2013;54(4):683–7.
Richon VM. Targeting histone deacetylases: development of vorinostat for the treatment of cancer. Epigenomics. 2010;2(3):457–65.
Mitsiades N. A road map to comprehensive androgen receptor axis targeting for castration-resistant prostate cancer. Cancer Res. 2013;73(15):4599–605.
Rao RD, Cobleigh MA. Adjuvant endocrine therapy for breast cancer. Oncology. 2012;26(6):541–7, 50, 52 passim.
Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–57.
Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–46.
Dutcher JP. Recent developments in the treatment of renal cell carcinoma. Ther Adv Urol. 2013;5(6):338–53.
Liu G, Franssen E, Fitch MI, Warner E. Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol. 1997;15(1):110–5.
Thanki K, Gangwal RP, Sangamwar AT, Jain S. Oral delivery of anticancer drugs: challenges and opportunities. J Control Release. 2013;170(1):15–40.
Lacouture ME, Anadkat MJ, Bensadoun RJ, Bryce J, Chan A, Epstein JB, et al. Clinical practice guidelines for the prevention and treatment of EGFR inhibitor-associated dermatologic toxicities. Support Care Cancer. 2011;19(8):1079–95.
Kantarjian HM, Fojo T, Mathisen M, Zwelling LA. Cancer drugs in the United States: Justum Pretium—the just price. J Clin Oncol. 2013;31(28):3600–4.
Pfister DG. The just price of cancer drugs and the growing cost of cancer care: oncologists need to be part of the solution. J Clin Oncol. 2013;31(28):3487–9.
Shen C, Chien C-R, Geynisman DM, Smieliauskas F, Shih Y-CT. A review of economic impact of targeted oral anticancer medications. Expert Rev Pharmacoecon Outcomes Res. 2014;14(1):45-69
Rajaratnam G, Edwards J. Imatinib for chronic myeloid leukaemia: a NICE mess. Lancet. 2001;358(9296):1902.
Kefford RF. Drug treatment for melanoma: progress, but who pays? Med J Aust. 2012;197(4):198–9.
Drummond M, Evans B, LeLorier J, Karakiewicz P, Martin D, Tugwell P, et al. Evidence and values: requirements for public reimbursement of drugs for rare diseases—a case study in oncology. Can J Clin Pharmacol 2009 Summer;16(2):e273–81; discussion e82–4.
Whyte S, Pandor A, Stevenson M. Bevacizumab for metastatic colorectal cancer: a NICE single technology appraisal. PharmacoEconomics. 2012;30(12):1119–32.
Yeung K, Carlson JJ. Clinical and economic review of erlotinib in non-small-cell lung cancer. Expert Rev Pharmacoecon Outcomes Res. 2012;12(4):411–23.
Glanville J, Paisley S. Identifying economic evaluations for health technology assessment. Int J Technol Assess Health Care. 2010;26(4):436–40.
HIRU. Search Filters for MEDLINE in Ovid Syntax and the PubMed translation. [cited 2013 September 3]. http://hiru.mcmaster.ca/hiru/HIRU_Hedges_MEDLINE_Strategies.aspx.
BLS. Consumer Price Index. [cited 2013 November 14]. http://www.bls.gov/cpi/.
IMF. World Economic Outlook Database. [cited 2013 December 7]. http://www.imf.org/external/pubs/ft/weo/2009/02/weodata/index.aspx.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
NCI. Cancer Drug Information. [cited 2013 December 7]. http://www.cancer.gov/cancertopics/druginfo/alphalist.
Le QA, Hay JW. Cost-effectiveness analysis of lapatinib in HER-2-positive advanced breast cancer. Cancer. 2009;115(3):489–98.
Machado M, Einarson TR. Lapatinib in patients with metastatic breast cancer following initial treatment with trastuzumab: an economic analysis from the Brazilian public health care perspective. Breast Cancer (Dove Med Press). 2012;2012(4):173–82.
Ebara T, Ohno T, Nakano T. Quantitative medical cost-effectiveness analysis of molecular-targeting cancer drugs in Japan. Daru. 2013;21(1):40.
Delea TE, Tappenden P, Sofrygin O, Browning D, Amonkar MM, Karnon J, et al. Cost-effectiveness of lapatinib plus capecitabine in women with HER2+ metastatic breast cancer who have received prior therapy with trastuzumab. Eur J Health Econ. 2012;13(5):589–603.
Experts in Chronic Myeloid Leukemia, The price of drugs for chronic myeloid leukemia. (CML) is a reflection of the unsustainable prices of cancer drugs: from the perspective of a large group of CML experts. Blood. 2013;121(22):4439–42.
Breitscheidel L. Cost utility of allogeneic stem cell transplantation with matched unrelated donor versus treatment with imatinib for adult patients with newly diagnosed chronic myeloid leukaemia. J Med Econ. 2008;11(4):571–84.
Reed SD, Anstrom KJ, Li Y, Schulman KA. Updated estimates of survival and cost effectiveness for imatinib versus interferon-alpha plus low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukaemia. PharmacoEconomics. 2008;26(5):435–46.
Chen Z, Wang C, Xu X, Feng W. Cost-effectiveness study comparing imatinib with interferon-alpha for patients with newly diagnosed chronic-phase (CP) chronic myeloid leukemia (CML) from the Chinese public health-care system perspective (CPHSP). Value Health. 2009;12 Suppl 3:S85–8.
Hoyle M, Rogers G, Moxham T, Liu Z, Stein K. Cost-effectiveness of dasatinib and nilotinib for imatinib-resistant or -intolerant chronic phase chronic myeloid leukemia. Value Health. 2011;14(8):1057–67.
Ghatnekar O, Hjalte F, Taylor M. Cost-effectiveness of dasatinib versus high-dose imatinib in patients with Chronic Myeloid Leukemia (CML), resistant to standard dose imatinib—a Swedish model application. Acta Oncol. 2010;49(6):851–8.
Patel SR, Wong P. The efficacy of imatinib in unresectable/metastatic gastrointestinal stromal tumors. US Oncol Rev. 2009;5(1):61–4.
Sanon M, Taylor DC, Parthan A, Coombs J, Paolantonio M, Sasane M. Cost-effectiveness of 3-years of adjuvant imatinib in gastrointestinal stromal tumors (GIST) in the United States. J Med Econ. 2013;16(1):150–9.
Majer IM, Gelderblom H, van den Hout WB, Gray E, Verheggen BG. Cost-effectiveness of 3-year vs 1-year adjuvant therapy with imatinib in patients with high risk of gastrointestinal stromal tumour recurrence in the Netherlands; a modelling study alongside the SSGXVIII/AIO trial. J Med Econ. 2013;16(9):1106–19.
Mabasa VH, Taylor SC, Chu CC, Moravan V, Johnston K, Peacock S, et al. Verification of imatinib cost-effectiveness in advanced gastrointestinal stromal tumor in British Columbia (VINCE-BC study). J Oncol Pharmacy Pract. 2008;14(3):105–12.
Paz-Ares L, Garcia del Muro X, Grande E, Gonzalez P, Brosa M, Diaz S. Cost-effectiveness analysis of sunitinib in patients with metastatic and/or unresectable gastrointestinal stroma tumours (GIST) after progression or intolerance with imatinib. Clin Transl Oncol. 2008;10(12):831–9.
Contreras-Hernandez I, Mould-Quevedo JF, Silva A, Salinas-Escudero G, Villasis-Keever MA, Granados-Garcia V, et al. A pharmaco-economic analysis of second-line treatment with imatinib or sunitinib in patients with advanced gastrointestinal stromal tumours. Br J Cancer. 2008;98(11):1762–8.
Vitale A, Volk ML, Pastorelli D, Lonardi S, Farinati F, Burra P, et al. Use of sorafenib in patients with hepatocellular carcinoma before liver transplantation: a cost-benefit analysis while awaiting data on sorafenib safety. Hepatology. 2010;51(1):165–73.
Muszbek N, Shah S, Carroll S, McDonald H, Dale P, Maroun J, et al. Economic evaluation of sorafenib in the treatment of hepatocellular carcinoma in Canada. Curr Med Res Opin. 2008;24(12):3559–69.
Carr BI, Carroll S, Muszbek N, Gondek K. Economic evaluation of sorafenib in unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 2010;25(11):1739–46.
Camma C, Cabibbo G, Petta S, Enea M, Iavarone M, Grieco A, et al. Cost-effectiveness of sorafenib treatment in field practice for patients with hepatocellular carcinoma. Hepatology. 2013;57(3):1046–54.
ALA (American Lung Association). Lung Cancer Fact Sheet. 2013 [cited 2013 November 17]. http://www.lung.org/lung-disease/lung-cancer/resources/facts-figures/lung-cancer-fact-sheet.html.
Chouaid C, Le Caer H, Locher C, Dujon C, Thomas P, Auliac JB, et al. Cost-effectiveness of erlotinib versus chemotherapy for first-line treatment of non small cell lung cancer (NSCLC) in fit elderly patients participating in a prospective phase 2 study (GFPC 0504). BMC Cancer. 2012;12:301.
Chouaid C, Le Caer H, Corre R, Crequit J, Locher C, Falchero L, et al. Cost analysis of erlotinib versus chemotherapy for first-line treatment of non-small-cell lung cancer in frail elderly patients participating in a prospective phase 2 study (GFPC 0505). Clin Lung Cancer. 2013;14(2):103–7.
Wang S, Peng L, Li J, Zeng X, Ouyang L, Tan C, et al. A trial-based cost-effectiveness analysis of erlotinib alone versus platinum-based doublet chemotherapy as first-line therapy for Eastern Asian nonsquamous non-small-cell lung cancer. PloS One. 2013;8(3):e55917.
Vergnenegre A, Ray JA, Chouaid C, Grossi F, Bischoff HG, Heigener DF, et al. Cross-market cost-effectiveness analysis of erlotinib as first-line maintenance treatment for patients with stable non-small cell lung cancer. Clinicoecon Outcomes Res. 2012;4:31–7.
Cappuzzo F, Ciuleanu T, Stelmakh L, Cicenas S, Szczesna A, Juhasz E, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11(6):521–9.
Walleser S, Ray J, Bischoff H, Vergnenegre A, Rosery H, Chouaid C, et al. Maintenance erlotinib in advanced nonsmall cell lung cancer: cost-effectiveness in EGFR wild-type across Europe. Clinicoecon Outcomes Res. 2012;4:269–75.
Klein R, Wielage R, Muehlenbein C, Liepa AM, Babineaux S, Lawson A, et al. Cost-effectiveness of pemetrexed as first-line maintenance therapy for advanced nonsquamous non-small cell lung cancer. J Thorac Oncol. 2010;5(8):1263–72.
Lewis G, Peake M, Aultman R, Gyldmark M, Morlotti L, Creeden J, et al. Cost-effectiveness of erlotinib versus docetaxel for second-line treatment of advanced non-small-cell lung cancer in the United Kingdom. J Int Med Res. 2010;38(1):9–21.
Cromwell I, van der Hoek K, Melosky B, Peacock S. Erlotinib or docetaxel for second-line treatment of non-small cell lung cancer: a real-world cost-effectiveness analysis. J Thorac Oncol. 2011;6(12):2097–103.
Carlson JJ, Reyes C, Oestreicher N, Lubeck D, Ramsey SD, Veenstra DL. Comparative clinical and economic outcomes of treatments for refractory non-small cell lung cancer (NSCLC). Lung Cancer. 2008;61(3):405–15.
Araujo A, Parente B, Sotto-Mayor R, Teixeira E, Almodovar T, Barata F, et al. An economic analysis of erlotinib, docetaxel, pemetrexed and best supportive care as second or third line treatment of non-small cell lung cancer. Rev Port Pneumol. 2008;14(6):803–27.
Thongprasert S, Tinmanee S, Permsuwan U. Cost-utility and budget impact analyses of gefitinib in second-line treatment for advanced non-small cell lung cancer from Thai payer perspective. Asia Pac J Clin Oncol. 2012;8(1):53–61.
Bradbury PA, Tu D, Seymour L, Isogai PK, Zhu L, Ng R, et al. Economic analysis: randomized placebo-controlled clinical trial of erlotinib in advanced non-small cell lung cancer. J Natl Cancer Inst. 2010;102(5):298–306.
Cromwell I, van der Hoek K, Malfair Taylor SC, Melosky B, Peacock S. Erlotinib or best supportive care for third-line treatment of advanced non-small-cell lung cancer: a real-world cost-effectiveness analysis. Lung Cancer. 2012;76(3):472–7.
Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev. 2008;34(3):193–205.
Buti S, Bersanelli M, Sikokis A, Maines F, Facchinetti F, Bria E, et al. Chemotherapy in metastatic renal cell carcinoma today? A systematic review. Anticancer Drugs. 2013;24(6):535–54.
Remak E, Charbonneau C, Negrier S, Kim ST, Motzer RJ. Economic evaluation of sunitinib malate for the first-line treatment of metastatic renal cell carcinoma. J Clin Oncol. 2008;26(24):3995–4000.
Wu B, Dong B, Xu Y, Zhang Q, Shen J, Chen H, et al. Economic evaluation of first-line treatments for metastatic renal cell carcinoma: a cost-effectiveness analysis in a health resource-limited setting. PloS One. 2012;7(3):e32530.
Calvo Aller E, Maroto P, Kreif N, Gonzalez Larriba JL, Lopez-Brea M, Castellano D, et al. Cost-effectiveness evaluation of sunitinib as first-line targeted therapy for metastatic renal cell carcinoma in Spain. Clin Transl Oncol. 2011;13(12):869–77.
Benedict A, Figlin RA, Sandstrom P, Harmenberg U, Ullen A, Charbonneau C, et al. Economic evaluation of new targeted therapies for the first-line treatment of patients with metastatic renal cell carcinoma. BJU Int. 2011;108(5):665–72.
Purmonen T, Martikainen JA, Soini EJ, Kataja V, Vuorinen RL, Kellokumpu-Lehtinen PL. Economic evaluation of sunitinib malate in second-line treatment of metastatic renal cell carcinoma in Finland. Clin Ther. 2008;30(2):382–92.
Paz-Ares L, del Muro JG, Grande E, Diaz S. A cost-effectiveness analysis of sunitinib in patients with metastatic renal cell carcinoma intolerant to or experiencing disease progression on immunotherapy: perspective of the Spanish National Health System. J Clin Pharmacy Ther. 2010;35(4):429–38.
Hoyle M, Green C, Thompson-Coon J, Liu Z, Welch K, Moxham T, et al. Cost-effectiveness of sorafenib for second-line treatment of advanced renal cell carcinoma. Value Health. 2010;13(1):55–60.
Casciano R, Chulikavit M, Di Lorenzo G, Liu Z, Baladi JF, Wang X, et al. Economic evaluation of everolimus versus sorafenib for the treatment of metastatic renal cell carcinoma after failure of first-line sunitinib. Value Health. 2011;14(6):846–51.
Tam VC, Ko YJ, Mittmann N, Cheung MC, Kumar K, Hassan S, et al. Cost-effectiveness of systemic therapies for metastatic pancreatic cancer. Curr Oncol. 2013;20(2):e90–e106.
Casciano R, Chulikavit M, Perrin A, Liu Z, Wang X, Garrison LP. Cost-effectiveness of everolimus vs sunitinib in treating patients with advanced, progressive pancreatic neuroendocrine tumors in the United States. J Med Econ. 2012;15(Suppl 1):55–64.
IOM (Institute of Medicine). Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Washington, DC: The National Academies Press; 2013.
Shih YC, Ganz PA, Aberle D, Abernethy A, Bekelman J, Brawley O, et al. Delivering high-quality and affordable care throughout the cancer care continuum. J Clin Oncol. 2013;31(32):4151–7.
Cheema PK, Gavura S, Migus M, Godman B, Yeung L, Trudeau ME. International variability in the reimbursement of cancer drugs by publically funded drug programs. Curr Oncol. 2012;19(3):e165–76.
OECD. Cancer care: assuring quality to improve survival. OECD Health Policy Studies, OECD Publishing, 2013. http://dx.doi.org/10.1787/9789264181052-en.
Drummond M, Towse A. Is it time to reconsider the role of patient co-payments for pharmaceuticals in Europe? Eur J Health Econ. 2012;13(1):1–5.
Valachis A, Polyzos NP, Nearchou A, Lind P, Mauri D. Financial relationships in economic analyses of targeted therapies in oncology. J Clin Oncol. 2012;30(12):1316–20.
Danzon PM, Furukawa MF. International prices and availability of pharmaceuticals in 2005. Health Aff (Millwood). 2008;27(1):221–33.
Squires DA. The U.S. health system in perspective: a comparison of twelve industrialized nations. Issue Brief (Commonw Fund). 2011;16:1–14
IOM (Institute of Medicine). Observational Studies in a Learning Health System: Workshop Summary. Washington, DC: The National Academies Press; 2013.
Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-effectiveness in health and medicine. Oxford: Oxford University Press; 1996.
American Cancer Society. Cancer Facts & Figures 2013. Atlanta, GA: American Cancer Society; 2013.
Ma X, Does M, Raza A, Mayne ST. Myelodysplastic syndromes: incidence and survival in the United States. Cancer. 2007;109(8):1536–42.
Gillick MR. Controlling off-label medication use. Ann Intern Med. 2009;150(5):344–7.
Mehta SS. Commercializing successful biomedical technologies. New York, NY: Cambridge University Press; 2008.
Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. 3rd ed. New York: Oxford University Press; 2005.
Zafar SY, Abernethy AP. Financial toxicity, Part II: how can we help with the burden of treatment-related costs? Oncology. 2013;27(4):253–4, 6.
Zafar SY, Abernethy AP. Financial toxicity, Part I: a new name for a growing problem. Oncology. 2013;27(2):80–1, 149.
Zafar SY, Peppercorn JM, Schrag D, Taylor DH, Goetzinger AM, Zhong X, et al. The financial toxicity of cancer treatment: a pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience. Oncologist. 2013;18(4):381–90.
IOM (Institute of Medicine). Facilitating collaborations to develop combination investigational cancer therapies: workshop summary. Washington, DC: The National Academies Press; 2012.
Acknowledgments
Drs. Shih and Smieliauskas are supported by a grant from the Agency for Healthcare Research and Quality (R01 HS018535), and Dr. Shih is also supported by The University of Chicago Cancer Research Foundation Women’s Board. Dr. Chien is supported by a grant from the Ministry of Health and Welfare (MOHW 103-TD-B-111-03). Dr. Geynisman acknowledges receiving honoraria for speaking from Pfizer. No other potential conflict of interest relevant to this article was reported.
Author Contribution
Drs. Smieliauskas and Chien drafted the manuscript, with the assistance of Dr. Geynisman on the paragraphs that provided the clinical background of cancer drugs. Drs. Smieliauskas, Chien, and Shen drafted the tables. Drs. Chien, Shih, and Geynisman determined search strategies, Dr. Chien conducted the literature search, and Drs. Chien, Shen, and Shih identified the articles included in the review. Dr. Shih supervised the overall progress and provided final revision, review, and comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
F. Smieliauskas and C.-R. Chien are joint first authors.
Rights and permissions
About this article
Cite this article
Smieliauskas, F., Chien, CR., Shen, C. et al. Cost-Effectiveness Analyses of Targeted Oral Anti-Cancer Drugs: A Systematic Review. PharmacoEconomics 32, 651–680 (2014). https://doi.org/10.1007/s40273-014-0160-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-014-0160-z