Abstract
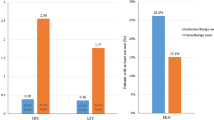
The arrival of personalized medicine in the clinic means that treatment decisions will increasingly rely on test results. The challenge of limited healthcare resources means that the dissemination of these technologies will be dependent on their value in relation to their cost, i.e., their cost effectiveness. Phelps and Mushlin have described how to optimize tests to meet a cost-effectiveness target. However, when tests are applied repeatedly the case mix of the patients tested changes with each administration, and this impacts upon the value of each subsequent test administration. In this article, we present a modification of Phelps and Mushlin’s framework for diagnostic tests; to identify the cost-effective cut-off for monitoring tests. Using the Ca125 test monitoring for relapse in ovarian cancer, we show how the repeated use of the initial cut-off can lead to a substantially increased false-negative rate compared with the monitoring cut-off—over 4 % higher than in this example—with the associated harms for individual and population health.



Similar content being viewed by others
References
National Institute for Health and Clinical Excellence. Diagnostics Assessment Programme. http://www.nice.org.uk/diagnostics. Accessed 29th July 2013.
Department of Health and Aging. Co-dependent and Hybrid Technologies. http://www.health.gov.au/internet/hta/publishing.nsf/Content/co-1. Accessed 29th July 2013.
Phelps CE, Mushlin A. Focussing technology assessment using decision theory. Med Decis Mak. 1988;8(4):279–89.
Guttmacher AE, Collins FS. Genomic Medicine: a primer. New Engl J Med. 2002;347(19):1512–20.
Carroll PR, Whitson JM, Cooperberg MR. Serum prostate-specific antigen for the early detection of prostate cancer: always, never, or only sometimes? J Clin Oncol. 2011;28(4):345–6.
Rustin GJ, van der Burg ME, Griffin CL, Guthrie D, Lamont A, Jayson GC, Kristensen G, Mediola C, Coens C, Qian W, Parmar MK. Swart AM; MRC OV05; EORTC 55955 investigators. Early versus delayed treatment of relapsed ovarian cancer (MRC OV05/EORTC 55955): a randomised trial. Lancet. 2010;376(9747):1155–63.
Parkes J, Guha IN, Roderick P, Harris S, Cross R, Manos MM, Irving W, Zaitoun A, Wheatley M, Ryder S, Rosenberg W. Enhanced liver fibrosis (ELF) test accurately identifies liver fibrosis in patients with chronic hepatitis C. J Viral Hepat. 2011;18(1):23–31. doi:10.1111/j.1365-2893.2009.01263.x.
Hosmer DW, Lemeshow S. Applied logistic regression. 2nd ed. New York: Wiley; 2000. p. 156–164.
McCabe C, Claxton K, Culyer AJ. The NICE cost effectiveness threshold: what it is and what that means? PharmacoEconomics. 2008;26(9):733–44.
NICE. Guide to the Methods of Technology Appraisal. London; 2008.
Pesce LL, Metz CE, Berbaum KS. On the convexity of ROC curves estimated from radiological test results. Acad Radiol. 2010;17(8):960–8.
Grey DR, Morgan BJT. Some aspects of ROC curve-fitting: normal and logistic models. J Math Psychol. 1972;9:128–39.
Gu W, Pepe MS. Estimating the diagnostic likelihood ratio of a continuous marker. Biostatistics. 2011;12(1):87–101.
Hanley JS. The robustness of the binormal assumptions used in fitting ROC curves. Med Decis Mak. 1988;8:197–203.
Metz CE, Herman BA, Shen J-H. Maximum-likelihood estimation of ROC curves from continuously-distributed data. Stat Med. 1998;17:1033–53.
Metz CE, Pan X. Proper binormal ROC curves: theory and maximum-likelihood estimation. J Math Psychol. 1999;43:1–33.
Burden RL, Faires JD. Numerical analysis. 7th ed. Conn: Brooks/Cole; 2000.
Pesce LL, Metz CE. Reliable and computationally efficient maximum-likelihood estimation of “proper” binormal ROC curves. Acad Radiol. 2007;14:814–29.
National Institute for Health and Clinical Excellence. Ovarian cancer: the recognition and initial management of ovarian cancer. London: NICE Full Guideline; 2011.
Kind P, Dolan P, Gudex C, Williams A. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ. 1998;316:736–41.
Mason H, Jones-Lee M, Donaldson C. Modelling the monetary value of a QALY: a new approach based on UK data. Health Econ. 2009;18(8):933–50.
Towse A. Should NICE’s threshold range for cost per QALY be raised? Yes. BMJ. 2009;338:268–9.
Claxton K, Martin P, Soares M, et al. Methods for the estimation of the NICE cost effectiveness threshold. Draft Final Report October 2012. http://www.york.ac.uk/media/che/documents/reports/Methods%20for%20the%20Estimation%20of%20the%20NICE%20Cost%20Effectiveness%20Threshold%20%28Draft%20Final%20Report%29%20%281%29.pdf.
Novielli N, Cooper NJ, Sutton AJ. Evaluating the cost-effectiveness of diagnostic tests in combination: is it important to allow for performance dependency? Value Health. 2013;16(4):536–41.
Acknowledgments
This article presents independent research partially funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research Programme (RP-PG-0707-10101: Evaluating the benefits for patients and the NHS of new and existing biological fluid biomarkers in liver and renal disease). Additional support was received through the Medical Technologies Innovation and Knowledge Centre funded by the UK Research Councils, EPSRC, and BBSRC, and the Technology Strategy Board. We wish to acknowledge helpful discussions with colleagues on the NIHR Programme Grant award, especially Doug Altman, Jon Deeks, Walter Gregory, and Peter Selby. Prof. McCabe’s research is made possible by the Capital Health Research Chair Endowment to the University of Alberta Faculty of Medicine and Dentistry. He also received funding from the Genome Canada Large Scale Applied Research Program on Personalized Medicine for some of this work. The work reported in this paper and any errors remain the responsibility of the authors.
All individuals who made substantive contributions to the ideas presented in this article are included as authors. The initial concepts for this article were proposed by Christopher McCabe. Roberta Longo and Paul Baxter undertook the modeling work to provide the example and prepared the draft for the manuscript. Peter Hall, Geoff Hall, and Mehran Afshar provided the clinical data and contributed to the manuscript writing. Christopher McCabe and Jenny Hewison supervised the preparation of the draft and contributed to the manuscript writing.
Conflicts of interest
Roberta Longo has no conflicts of interest.
Paul Baxter has no conflicts of interest.
Peter Hall has no conflicts of interest.
Jenny Hewison has no conflicts of interest.
Mehran Afshar has no conflicts of interest.
Geoff Hall has no conflicts of interest.
Christopher McCabe has no conflicts of interest.
Disclaimer
The views and opinions expressed by the authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the Department of Health, or Genome Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Longo, R., Baxter, P., Hall, P. et al. Methods for Identifying the Cost-effective Case Definition Cut-Off for Sequential Monitoring Tests: An Extension of Phelps and Mushlin. PharmacoEconomics 32, 327–334 (2014). https://doi.org/10.1007/s40273-014-0134-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-014-0134-1