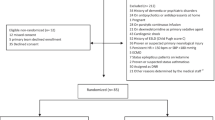
Abstract
Background
Ketamine has been considered as an adjunct for children who do not reach their predefined target sedation depth. However, there is limited evidence regarding the use of ketamine as a prolonged infusion (i.e., >24 hours) in the pediatric intensive care unit (PICU).
Objective
We sought to evaluate the safety and effectiveness of continuous ketamine infusion for >24 hours in mechanically ventilated children.
Methods
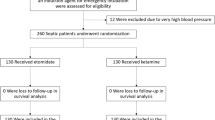
We conducted a prospective cohort study in a tertiary PICU from January 2020 to December 2022. The primary outcome was the incidence of adverse events (AEs) after ketamine initiation. The secondary outcome included assessing the median proportion of time the patient spent on the Richmond Agitation-Sedation Scale (RASS) goal after ketamine infusion. Patients were also divided into two groups based on the sedative regimen, ketamine-based or non-ketamine-based, to assess the incidence of delirium.
Results
A total of 269 patients were enrolled: 73 in the ketamine group and 196 in the non-ketamine group. The median infusion rate of ketamine was 1.4 mg/kg/h. Delirium occurred in 16 (22%) patients with ketamine and 15 (7.6%) patients without ketamine (p = 0.006). After adjusting for covariates, logistic regression showed that delirium was associated with comorbidities (odds ratio [OR] 4.2), neurodevelopmental delay (OR 0.23), fentanyl use (OR 7.35), and ketamine use (OR 4.17). Thirty-one (42%) of the patients experienced at least one AE following ketamine infusion. Other AEs likely related to ketamine were hypertension (n = 4), hypersecretion (n = 14), tachycardia (n = 6), and nystagmus (n = 2). There were no significant changes in hemodynamic variables 24 h after the initiation of ketamine. Regarding the secondary outcomes, patients were at their goal RASS level for a median of 76% (range 68–80.5%) of the time in the 24 hours before ketamine initiation, compared with 84% (range 74.5–90%) of the time during the 24 h after ketamine initiation (p < 0.001). The infusion rate of ketamine did not significantly affect concomitant analgesic and sedative infusions. The ketamine group experienced a longer duration of mechanical ventilation and a longer length of stay in the PICU and hospital than the non-ketamine group.
Conclusion
The use of ketamine infusion in PICU patients may be associated with an increased rate of adverse events, especially delirium. High-quality studies are needed before ketamine can be broadly recommended or adopted earlier in the sedation protocol.

Similar content being viewed by others
Data Availability
This published article includes all data generated or analyzed during this study.
Code Availability
Not applicable.
References
Manasco AT, Stephens RJ, Yaeger LH, et al. Ketamine sedation in mechanically ventilated patients: a systematic review and meta-analysis. J Crit Care. 2020;56:80–8.
Smith HAB, Besunder JB, Betters KA, et al. 2022 society of critical care medicine clinical practice guidelines on prevention and management of pain, agitation, neuromuscular blockade, and delirium in critically Ill pediatric patients with consideration of the icu environment and early mobility. Pediatr Crit Care Med. 2022;23(2):e74–110.
Amigoni ACG, Conio A, Corno M, et al. Recommendations for analgesia and sedation in critically ill children admitted to intensive care unit. J Anesth Analg Crit Care. 2022;12(2):9.
Daverio M, von Borell F, Ramelet AS, et al. Pain and sedation management and monitoring in pediatric intensive care units across Europe: an ESPNIC survey. Crit Care. 2022;26(1):88.
Garber PM, Droege CA, Carter KE, et al. Continuous infusion ketamine for adjunctive analgosedation in mechanically ventilated. Critically Ill Patients. Pharmacotherapy. 2019;39(3):288–96.
Patanwala AE, Martin JR, Erstad BL. Ketamine for analgosedation in the intensive care unit: a systematic review. J Intensive Care Med. 2017;32(6):387–95.
Park S, Choi AY, Park E, et al. Effects of continuous ketamine infusion on hemodynamics and mortality in critically ill children. PLoS One. 2019;14(10): e0224035.
Tessari A, Sperotto F, Pece F, et al. Is ketamine infusion effective and safe as an adjuvant of sedation in the PICU? Results Ketamine Infus Sedation Study (KISS). 2023;43(7):622–31.
Sperotto F, Giaretta I, Mondardini MC, et al. Ketamine prolonged infusions in the pediatric intensive care unit: a tertiary-care single-center analysis. J Pediatr Pharmacol Ther. 2021;26(1):73–80.
Heiberger AL, Ngorsuraches S, Olgun G, et al. Safety and utility of continuous ketamine infusion for sedation in mechanically ventilated pediatric patients. J Pediatr Pharmacol Ther. 2018;23(6):447–54.
Johnson PN, Mayes R, Moore E, et al. Ketamine infusions as an adjunct for sedation in critically ill children. J Opioid Manag. 2022;18(1):57–68.
Harris J, Ramelet AS, van Dijk M, et al. Clinical recommendations for pain, sedation, withdrawal and delirium assessment in critically ill infants and children: an ESPNIC position statement for healthcare professionals. Intensive Care Med. 2016;42(6):972–86.
Kerson AG, DeMaria R, Mauer E, et al. Validity of the richmond agitation-sedation scale (RASS) in critically ill children. J Intensive Care. 2016;4:65.
Merkel SI, Voepel-Lewis T, Shayevitz JR, et al. The FLACC: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997;23(3):293–7.
Edwards JD, Houtrow AJ, Vasilevskis EE, et al. Chronic conditions among children admitted to U.S. pediatric intensive care units: their prevalence and impact on risk for mortality and prolonged length of stay. Crit Care Med. 2012;40(7):2196–203.
Pollack MM, Ruttimann UE, Getson PR. Pediatric risk of mortality (PRISM) score. Crit Care Med. 1988;16(11):1110–6.
Leteurtre S, Martinot A, Duhamel A, et al. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet. 2003;362(9379):192–7.
Mokhlesi B, Tulaimat A, Gluckman TJ, et al. Predicting extubation failure after successful completion of a spontaneous breathing trial. Respir Care. 2007;52(12):1710–7.
Silver G, Traube C, Kearney J, et al. Detecting pediatric delirium: development of a rapid observational assessment tool. Intensive Care Med. 2012;38(6):1025–31.
Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–45.
Golding CL, Miller JL, Gessouroun MR, et al. Ketamine continuous infusions in critically Ill infants and children. Ann Pharmacother. 2016;50(3):234–41.
Siegel EJ, Traube C. Pediatric delirium: epidemiology and outcomes. Curr Opin Pediatr. 2020;32(6):743–9.
Ista E, Traube C, de Neef M, et al. Factors associated with delirium in children: a systematic review and meta-analysis. Pediatr Crit Care Med. 2023;24(5):372–81.
Sperotto F, Ramelet AS, Daverio M, et al. Assessment and management of iatrogenic withdrawal syndrome and delirium in pediatric intensive care units across Europe: an ESPNIC survey. Pharmacotherapy. 2023;43(8):804–15.
Shurtleff V, Radosevich JJ, Patanwala AE. Comparison of ketamine- versus nonketamine-based sedation on delirium and coma in the intensive care unit. J Intensive Care Med. 2020;35(6):536–41.
Chan K, Burry LD, Tse C, et al. Impact of ketamine on analgosedative consumption in critically Ill patients: a systematic review and meta-analysis. Ann Pharmacother. 2022;56(10):1139–58.
Flores AER, Oura KHU, Rocha PK, et al. Incidence and factors associated with delirium in children in a single pediatric intensive care unit in Brazil. J Pediatr Nurs. 2021;61:e29–34.
Gupta N, Woolley A, Talathi S, et al. Opioid use is associated with ICU delirium in mechanically ventilated children. J Crit Care Med (Targu Mures). 2020;6(3):167–74.
Ricardo Ramirez C, Alvarez Gomez ML, Agudelo Velez CA, et al. Clinical characteristics, prevalence, and factors related to delirium in children of 5–14 years of age admitted to intensive care. Med Intensiva (Engl Ed). 2019;43(3):147–55.
Funding
No funding was received to prepare this article.
Author information
Authors and Affiliations
Contributions
PSLS and MCMF conceived and designed the research; PSLS and MCMF had direct clinical responsibility for the patients; EYK and RMRS participated in data acquisition and curation; PSLS, EYK, and RMRS performed the data collection; PSLS and MCMF performed the statistical analysis; PSLS and MCMF wrote the original draft; EYK and RMRS critically reviewed the scientific content of the manuscript; all authors revised, read, and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Paulo Sérgio Lucas da Silva, Emerson Yukio Kubo, Rafael da Motta Ramos Siqueira, and Marcelo Cunio Machado Fonseca have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
Hospital do Servidor Publico Municipal review board approved the study (IRB P-00036098).
Informed Consent
Written informed consent was obtained from both parents.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
da Silva, P.S.L., Kubo, E.Y., da Motta Ramos Siqueira, R. et al. Impact of Prolonged Continuous Ketamine Infusions in Critically Ill Children: A Prospective Cohort Study. Pediatr Drugs (2024). https://doi.org/10.1007/s40272-024-00635-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s40272-024-00635-9