Abstract
Purpose
The purpose of this study is to describe medications most commonly studied in pediatric polypharmacy research by pharmacologic classes and disease using a scoping review methodology.
Methods
A search of electronic databases was conducted in July 2019 that included Ovid Medline, PubMed, Elsevier Embase, and EBSCO CINAHL. Primary observational studies were selected if they evaluated polypharmacy as an aim, outcome, predictor, or covariate in children 0–21 years of age. Studies not differentiating between adults and children or those not written in English were excluded. Study characteristics, pharmacologic categories, medication classes, and medications were extracted from the included studies.
Results
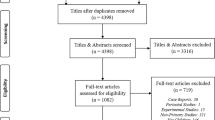
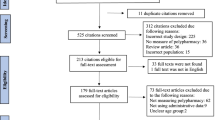
The search identified 8790 titles and after de-duplicating and full-text screening, 414 studies were extracted for the primary data. Regarding global pharmacologic categories, central nervous system (CNS) agents were most studied (n = 185, 44.9%). The most reported pharmacologic category was the anticonvulsants (n = 250, 60.4%), with valproic acid (n = 129), carbamazepine (n = 123), phenobarbital (n = 87), and phenytoin (n = 83) being the medications most commonly studied. In studies that reported medication classes (n = 105), serotonin reuptake inhibitors (n = 32, 30.5%), CNS stimulants (n = 30, 28.6%), and mood stabilizers (n = 27, 25.7%) were the most studied medication classes.
Conclusion
While characterizing the literature on pediatric polypharmacy in terms of the types of medication studied, we further identified substantive gaps within this literature outside of epilepsy and psychiatric disorders. Medications frequently identified in use of polypharmacy for treatment of epilepsy and psychiatric disorders reveal opportunities for enhanced medication management in pediatric patients.



Similar content being viewed by others
References
Ahmed B, Nanji K, Mujeeb R, Patel MJ. Effects of polypharmacy on adverse drug reactions among geriatric outpatients at a tertiary care hospital in Karachi: a prospective cohort study. PLoS One. 2014;9(11):e112133. https://doi.org/10.1371/journal.pone.0112133.
Gnjidic D, Hilmer SN, Blyth FM, Naganathan V, Waite L, Seibel MJ, et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J Clin Epidemiol. 2012;65(9):989–95.
Helal SI, Megahed HS, Salem SM, Youness ER. Monotherapy versus polytherapy in epileptic adolescents. Maced J Med Sci. 2013;6(2):174–7.
Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017. https://doi.org/10.1186/s12877-017-0621-2.
Poudel P, Chitlangia M, Pokharel R. Predictors of poor seizure control in children managed at a tertiary care hospital of Eastern Nepal. Iran J Child Neurol. 2016;10(3):48.
Rasu RS, Iqbal M, Hanifi S, Moula A, Hoque S, Rasheed S, et al. Level, pattern, and determinants of polypharmacy and inappropriate use of medications by village doctors in a rural area of Bangladesh. ClinicoEcon Outcomes Res. 2014;CEOR(6):515–21.
Viktil KK, Blix HS, Moger TA, Reikvam A. Polypharmacy as commonly defined is an indicator of limited value in the assessment of drug-related problems. Br J Clin Pharm. 2006;63(2):187–95.
Bakaki P, Horace A, Dawson N, et al. Defining pediatric polypharmacy: a scoping review. PLoS One. 2018;13(11):e0208047. https://doi.org/10.1371/journal.pone.0208047.
Feudtner C, Dai D, Hexem KR, Luan X, Metjian TA. Prevalence of polypharmacy exposure among hospitalized children in the United States. Arch Pediatr Adolesc Med. 2012;166(1):9–16.
Feinstein J, Dai D, Zhong W, Freedman J, Feudtner C. Potential drug–drug interactions in infant, child, and adolescent patients in children’s hospitals. Pediatrics. 2015;135(1):399-e108.
Kurian J, Mathew J, Sowjanya K, et al. Adverse drug reactions in hospitalized pediatric patients: a prospective observational study. Indian J Pediatr. 2016;83(5):414–9.
Dai D, Feinstein J, Morrison W, et al. Epidemiology of polypharmacy and potential-drug–drug interactions among pediatric patients in intensive care units of US children’s hospitals. Pediatr Crit Care Med. 2016;17(5):e2018–288.
Goldberg JF, Brooks JO, Kurita K, et al. Depressive illness burden associated with complex polypharmacy in patients with bipolar disorder: findings from the STEP-BD. J Clin Psychiatr. 2009;70(2):155–62.
Preskorn SH, Lacey RL. Polypharmacy: when is it rational? J Psychiatr Pract. 2007;13(2):97–105.
Fontanella CA, Warner LA, Phillips GS, Bridge JA, et al. Trends in psychotropic polypharmacy among youths enrolled in Ohio Medicaid, 2002–2008. Psychiatr Serv. 2014;65(11):1332–40.
Procyshyn RM, Su J, Eibe D, Liu AY, Panenka WJ, et al. Prevalence and patterns of antipsychotic use in youth at the time of admission and discharge from an inpatient psychiatric facility. J Clin Psychopharmacol. 2014;34(1):17–22.
Lohr DW, Creel L, Feygin Y, et al. Psychotropic polypharmacy among children and youth receiving Medicaid, 2012–2016. J Manag Spec Pharm. 2018;24(8):736–44.
Soria SR, Lui X, Hincapie-Castillo JM, Zambrano D, et al. Prevalence, time, trends, and utilization patterns of psychotropic polypharmacy among pediatric Medicaid beneficiaries, 1999–2010. Psychiatr Serv. 2018;69(8):919–26.
Olfson M, Banco C, Lui L, et al. National trends in outpatient treatment of children and adolescents with antipsychotic drugs. Arch Gen Psychiatr. 2006;63:679–85.
Lagerberg T, Molero Y, D’Onofrio BM, et al. Antidepressant prescription patterns and CNS polypharmacy with antidepressants among children, adolescents, and young adults: a population-based study in Sweden. Eur Child Adolesc Psychiatr. 2018. https://doi.org/10.1007/s00787-018-01269-2.
Dharni A, Coates D. Psychotropic medication profile in a community youth mental health service in Australia. Child Youth Serv Rev. 2018;90:8–14.
Senthilselvi R, Boopana M, Sthyan L, et al. Drug utilization pattern in paediatric patients in a secondary care hospital. Int J Pharm Pharm Sci. 2019;11:69–74.
National Survey of Children’s Health (2016–2018). In: Data resource center for child and adolescent health. https://www.childhealthdata.org/browse/survey?s=2&y=28&r=1. Accessed 31 Oct 2019.
Hoon D, Taylor MT, Kapadia P, Gerhard T, et al. Trends in off-label drug use in ambulatory settings: 2006–2015. Pediatrics. 2019;144(4):1–10.
Kern S. Challenges in conducting clinical trials in children: approaches for improving performance. Expert Rev Clin Pharmacol. 2009;2(6):609–17.
Arksey H, O’Malley LO. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32.
Colquhoun HL, Levac D, O’Brien KK, Straus S, Tricco AC, Perrier L, et al. Scoping reviews: time for clarity in definition, methods, and reporting. J Clin Epidemiol. 2014;67(12):1291–4.
Daudt HM, van Mossel C, Scott SJ. Enhancing the scoping study methodology: a large, inter-professional team’s experience with Arksey and O’Malley’s framework. BMC Med Res Methodol. 2013;13(48):1–9.
Peters MD, Godfrey CM, Khalil H, McInerney P, et al. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13(3):141–6.
Levac D, Colquhoun H, Brien KKO. Scoping studies: advancing the methodology. Implement Sci. 2010;5(69):1–9.
Pham MT, Rajić A, Greig JD, Sargeant JM, Papadopoulos A, McEwen SA. A scoping review of scoping reviews: advancing the approach and enhancing the consistency. Res Synth Methods. 2014;5(4):371–85.
Khalil H, Peters M, Godfrey CM, Mcinerney P, Soares CB, Parker D. An evidence-based approach to scoping reviews. Worldviews Evid Based Nurs. 2016;13(2):118–23.
Bakaki P, Staley J, Liu R, Dawson N, Golchin N, Horace A, et al. A transdisciplinary team approach to scoping reviews: the case of pediatric polypharmacy. BMC Med Res Methodol. 2018;18(1):102.
American Society of Health System Pharmacists. American Hospital Formulary Services Pharmacologic—Therapeutic Classification. 2018. https://www.ahfsdruginformation.com/ahfs-pharmacologic-therapeutic-classification/#1455219636269-43966bc3-86a3. Accessed 1 Nov 2018.
Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;307(7):758–66.
Donnelly JP, Baddley JW, Wang HE. Antibiotic utilization for acute respiratory tract infections in US emergency departments. Antimicrob Agents Chemother. 2014;58(3):1451–7.
Fleming-Dutra KE, Hersh AL, Shapiro DJ, Bartoces M, Enns EA, File TM, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA. 2016;315:1864–73.
Torpy J, Campbell A, Glass R. Chronic diseases of children. JAMA. 2010;303(7):682.
Patsalos PN. Drug interactions with the newer antiepileptic drugs (AEDs)—Part 2: pharmacokinetic and pharmacodynamic interactions between AEDs and drugs used to treat non-epilepsy disorders. Clin Pharmacokinet. 2013;52(12):1045–61.
Balan S, Hassali MA, Mak VSL. Challenges in pediatric drug use: a pharmacist point of view. Res Social Adm Pharm. 2017;13(3):653–5.
Gallego JA, Nielsen J, De Hert M, Kane JM, Correll CU. Safety and tolerability of antipsychotic polypharmacy. Expert Opin Drug Saf. 2012;11(4):527.
Lochmann van Bennekom MW, Gijsman HJ, Zitman FG. Antipsychotic polypharmacy in psychotic disorders: a critical review of neurobiology, efficacy, tolerability and cost effectiveness. J Psychopharmacol. 2013;27(4):327–36.
Sander JW, Perucca E. Epilepsy and comorbidity: infections and antimicrobials usage in relation to epilepsy management. Acta Neurol Scan Suppl. 2003;180:16–22.
Miranda MJ, Ahmad BB. Treatment of rolandic epilepsy. Ugeskr Laeger 2017;179(48):V06170482.
Van Ool JS, Snoeijen-Schouwenaars FM, Schelhaas HJ, Tan IY, Aldenkamp AP, Hendriksen JGM. A systematic review of neuropsychiatric comorbidities in patients with both epilepsy and intellectual disability. Epilepsy Behav. 2016;60:130–7.
Verrotti A, Moavero R, Panzarino G, Di Paolantonio C, Rizzo R, Curatolo P. The challenge of pharmacotherapy in children and adolescents with epilepsy-ADHD comorbidity. Clin Drug Investig. 2018;38(1):1–8.
Fedorowicz VJ, Fombonne E. Metabolic side effects of atypical antipsychotics in children: a literature review. J Psychopharmacol. 2005;19(5):533–50.
Andrade SE, Lo JC, Roblin D, Fouayzi H, Connor DF, Penfold RB, et al. Antipsychotic medication used among children and risk of diabetes mellitus. Pediatrics. 2011;128:1135–41.
Braüner JV, Johansen LM, Roesbjerg T, Pagsberg AK. Off-label prescription of psychopharmacological drugs in child and adolescent psychiatry. J Clin Psychopharmacol. 2016;36:500–7.
Olfson M, King M, Schoenbaum M. Treatment of young people with antipsychotic medications in the United States. JAMA Psychiatry. 2015;72(9):867–74.
Rutecki PA, Gidal BE. Antiepileptic drug treatment in the developmentally disabled: treatment considerations with the newer antiepileptic drugs. Epilepsy Behav. 2002;3(6S1):24–31.
Belousova ED. Perampanel in treatment of refractory partial epilepsy in adolescents and adults: results of international multicenter randomized, double-blind, placebo-controlled phase III studies. Zh Nevrol Psikhiatr Im S S Korsakova. 2014;114(8):32–8.
Ilies D, Huet AS, Lacourse E, Roy G, Stip E, Amor LB. Long-term metabolic effects in French-Canadian children and adolescents treated with second-generation antipsychotics in monotherapy or polytherapy: a 24-month descriptive retrospective study. Can J Psychiatry. 2017;62(12):827–36.
Plevin P, Jureidini J, Howell S, Smith N. Paediatric antiepileptic polytherapy: systematic review of efficacy and neurobehavioral effects and a tertiary centre experience. Acta Paediatr. 2018. https://doi.org/10.1111/apa.14343.
Anderson M, Egunsola O, Cherrill J, Millward C, Choonara I. A prospective study of adverse drug reactions to antiepileptic drugs in children. BMJ Open. 2015;596:008298. https://doi.org/10.1136/bmjopen-2015-008298.
Rashed AN, Wilton L, Lo CC, Kwong BY, Leung S, Wong IC. Epidemiology and potential risk factors of drug-related problems in Hong Kong paediatric wards. BJCP. 2014;77(5):873–9.
Rashed AN, Neubert A, Tomlin S, et al. Epidemiology and potential associated risk factors of drug-related problems in hospitalised children in the United Kingdom and Saudi Arabia. Eur J Clin Pharmacol. 2012;68(12):1657–66.
Schwartz EJ, Turgeon J, Patel J, Patel P, Shah H, Issa AM, et al. Implementation of a standardized medication therapy management plus approach within primary care. J Am Board Fam Med. 2017;30(6):701–14.
Wittayanukorn S, Westrick SC, Hansen RA, et al. Evaluation of medication therapy management services for patients with cardiovascular disease in a self-insured employer health plan. J Manag Care Pharm. 2013;19(5):385–95.
Ramalho de Oliveira D, Brummel AR, Miller DB. Medication therapy management: 10 years of experience in a large integrated health care system. J Manag Care Pharm. 2010;16(3):185–95.
American College of Clinical Pharmacy. Leadership for Medication Management. https://www.accp.com/docs/govt/advocacy/Leadership%20for%20Medication%20Management%20-%20MTM%20101.pdf. Accessed 23 Jan 2019.
Acknowledgements
We thank our expert stakeholders whose contribution at different stages of the project improved our research protocol, data quality, interpretation, and reporting: Dr. Joseph Calabrese, Dr. Faye Gary, Dr. Cynthia Fontanella, and Dr. Mai Pham. Thank you to Ms. Jennifer Staley, Dr. Sharon Meropol, Dr. Shari Bolen, and Dr. Almut Winterstein for their integral part in developing our methodology. We are also grateful to Ms. Courtney Baker and Ms. Rujia Liu, who conducted a great amount of study screening, data extraction, data cleaning, quality checks, processing, and analysis. Finally, we show appreciation to Ms. Tenerica Madison for her help in the development of our charts.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors, Alexis E. Horace, Negar Golchin, Elia M. Pestana Knight, Neal V. Dawson, Xuan Ma, James A. Feinstein, Hannah K. Johnson, Lawrence Kleinman, and Paul M. Bakaki, certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Funding
National Center for Advancing Translational Sciences (Clinical and Translational Science Collaborative of Cleveland): KL2TR002547; Eunice Kennedy Shriver National Institute for Child Health and Human Development: K23 HD091295.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Horace, A.E., Golchin, N., Knight, E.M.P. et al. A Scoping Review of Medications Studied in Pediatric Polypharmacy Research. Pediatr Drugs 22, 85–94 (2020). https://doi.org/10.1007/s40272-019-00372-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-019-00372-4