Abstract
Background
In adults, the area under the concentration–time curve (AUC) divided by the minimum inhibitory concentration (MIC) is associated with better clinical and bacteriological response to vancomycin in patients with methicillin-resistant Staphylococcus aureus who achieve target AUC/MIC ≥ 400. This target is often extrapolated to pediatric patients despite the lack of similar evidence. The impracticalities of calculating the AUC in practice means vancomycin trough concentrations are used to predict the AUC/MIC.
Objective
This review aimed to determine the relationship between vancomycin trough concentrations and AUC/MIC in pediatric patients.
Methods
We searched the MEDLINE and Embase databases, the Cochrane Database of Systematic Reviews, and the Cochrane Central Register of Controlled Trials using the medical subject heading (MeSH) terms vancomycin and AUC and pediatric* or paediatric*. Articles were included if they were published in English and reported a relationship between vancomycin trough concentrations and AUC/MIC.
Results
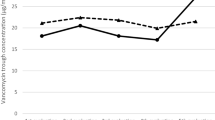
Of 122 articles retrieved, 11 met the inclusion criteria. One trial reported a relationship between vancomycin trough concentrations, AUC/MIC, and clinical outcomes but was likely underpowered. Five studies found troughs 6–10 mg/l were sufficient to attain an AUC/MIC > 400 in most general hospitalized pediatric patients. One study in patients undergoing cardiothoracic surgery found a trough of 18.4 mg/l achieved an AUC/MIC > 400. Two oncology studies reported troughs ≥ 15 mg/l likely attained an AUC/MIC ≥ 400. In critical care patients: one study found a trough of 9 mg/l did not attain the AUC/MIC target; another found 7 mg/l corresponded to an AUC/MIC of 400.
Conclusions
Potential vancomycin targets varied based on the population studied but, for general hospitalized pediatric patients, troughs of 6–10 mg/l are likely sufficient to achieve AUC/MIC ≥ 400. For MIC ≥ 2 mg/l, higher troughs are likely necessary to achieve an AUC/MIC ≥ 400. More research is needed to determine the relationships between vancomycin trough concentrations, AUC/MIC, and clinical outcomes.

Similar content being viewed by others
References
Reynolds P. Structure, biochemistry and mechanism of action of glycopeptide antibiotics. Eur J Clin Microbiol Infect Dis. 1989;8:943–50.
Levine DP. Vancomycin: a history. Clin Infect Dis. 2006;42:S5–12.
Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52.
Rybak MJ. The pharmacokinetic and pharmacodynamic properties of vancomycin. clinical infectious diseases [Internet]. 2006;42:S35–9. https://cid.oxfordjournals.org/lookup/doi/10.1086/491712.
Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004;43:925–42.
Rodvold KA, Blum RA, Fischer JH, et al. Vancomycin pharmacokinetics in patients with various degrees of renal function. Antimicrob Agents Chemother. 1988;32:848–52.
Pai MP, Neely M, Rodvold KA, Lodise T. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 2014;77:50–7.
Shipkova M, Petrova DT, Rosler AE, Orth M, Engelmayer J, Wieland E, et al. Comparability and imprecision of 8 frequently used commercially available immunoassays for therapeutic drug monitoring. therapeutic drug monitoring [Internet]. 2014;36:433–41. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00007691-201408000-00003.
McKamy S, Hernandez E, Jahng M, Moriwaki T, Deveikis A, Le J. Incidence and risk factors influencing the development of vancomycin nephrotoxicity in children. J Pediatr [Internet]. Mosby, Inc.; 2011;158:422–6. https://dx.doi.org/10.1016/j.jpeds.2010.08.019.
da Silva Alves GC, da Silva SD, Frade VP, Rodrigues D, Baldoni A de O, de Castro WV, et al. Determining the optimal vancomycin daily dose for pediatrics: a meta-analysis. Eur J Clin Pharmacol [Internet]. 2017. https://link.springer.com/10.1007/s00228-017-2306-3.
Hadi OA, Al Omar S, Nazer LH, Mubarak S, Le J. Vancomycin pharmacokinetics and predicted dosage requirements in pediatric cancer patients. J Oncol Pharm Pract [Internet]. 2015;22:448–53. http://opp.sagepub.com/content/22/3/448?etoc.
Benefield EC, Hagemann TM, Allen HC, Farmer K, Burton ME, Chavez-Bueno S, et al. Vancomycin dosing and pharmacokinetics in postoperative pediatric cardiothoracic surgery patients. J Pediatr Pharmacol Therapeut [Internet]. 2016;21:66–74. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4778698&tool=pmcentrez&rendertype=abstract.
Chhim RF, Arnold SR, Lee KR. Vancomycin dosing practices, trough concentrations, and predicted area under the curve in children with suspected invasive staphylococcal infections. J Pediatr Infect Dis Soc. 2013;2:259–62.
Demirjian A, Finkelstein Y, Nava-Ocampo A, Arnold A, Jones S, Monuteaux M, et al. A randomized controlled trial of a vancomycin loading dose in children. Pediatr Infect Dis J [Internet]. 2013;32:1217–23. http://www.ncbi.nlm.nih.gov/pubmed/23817340.
Frymoyer A, Hersh AL, Benet LZ, Guglielmo BJ. Current recommended dosing of vancomycin for children with invasive methicillin-resistant Staphylococcus aureus infections is inadequate. Pediatr Infect Dis J [Internet]. 2009;28:398–402. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3101254&tool=pmcentrez&rendertype=abstract.
Frymoyer A, Hersh AL, Coralic Z, Benet LZ, Joseph Guglielmo B. Prediction of vancomycin pharmacodynamics in children with invasive methicillin-resistant Staphylococcus aureus infections: a Monte Carlo simulation. Clin Therapeut [Internet]. Excerpta Medica Inc.; 2010;32:534–42. https://dx.doi.org/10.1016/j.clinthera.2010.03.005.
Frymoyer A, Guglielmo BJ, Hersh AL. Desired vancomycin trough serum concentration for treating invasive methicillin-resistant Staphylococcal infections. Pediatr Infect Dis J [Internet]. 2013;32:1077–9. http://www.ncbi.nlm.nih.gov/pubmed/23652479.
Giachetto G, Telechea H, Speranza N, Oyarzun M, Nanni L, Menchaca A. Vancomycin pharmacokinetic–pharmacodynamic parameters to optimize dosage administration in critically ill children. Pediatr Crit Care Med. 2011;12:e250–4.
Gomez DS, Campos EV, De Azevedo RP, Da Silva JM, Ferreira MC, Sanches-Giraud C, et al. Individualised vancomycin doses for paediatric burn patients to achieve PK/PD targets. Burns. 2013;39:445–50.
Hahn A, Frenck RWF Jr, Zou Y, Vinks AA. Validation of a pediatric population pharmacokinetic model for vancomycin. Ther Drug Monit. 2015;37:413–6.
Hahn A, Frenck RWF Jr, Allen-Staat M, Zou Y, Vinks AA. Evaluation of target attainment of vancomycin area under the curve in children with methicillin-resistant Staphylococcus aureus bacteremia. Ther Drug Monit. 2015;37:619–25.
Hwang D, Chiu NC, Chang L, Peng CC, Huang DTN, Huang FY, et al. Vancomycin dosing and target attainment in children. J Microbiol Immunol Infect [Internet]. Elsevier Taiwan LLC; 2015;6–11. https://dx.doi.org/10.1016/j.jmii.2015.08.027.
Janssen EJH, Välitalo PAJ, Allegaert K, De Cock RFW, Simons SHP, Sherwin CMT, et al. Towards rational dosing algorithms for vancomycin in neonates and infants based on population pharmacokinetic modeling. Antimicrob Agents Chemother. 2016;60:1013–21.
Lanke S, Yu T, Rower JE, Balch AH, Korgenski EK, Sherwin CM. AUC-guided vancomycin dosing in adolescent patients with suspected sepsis. J Clin Pharmacol. 2016;57:77–84.
Le J, Bradley JS, Murray W, Romanowski GL, Tran TT, Nguyen N, et al. Improved vancomycin dosing in children using area under the curve exposure. Pediatr Infect Dis J [Internet]. 2013;32:e155–63. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00006454-201304000-00015.
Nassar L, Hadad S, Gefen A, Shachor-Meyouha Y, Mashiach T, Krivoy NKI. Prospective evaluation of the dosing regimen of vancomycin in children of different weights. Basic Clin Pharmacol Toxicol [Internet]. 2011;109:132–3. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed10&NEWS=N&AN=70623258.
Ploessl C, White C, Manasco K. Correlation of a vancomycin pharmacokinetic model and trough serum concentrations in pediatric patients. Pediatr Infect Dis J [Internet]. 2015;34:e244-7. http://www.ncbi.nlm.nih.gov/pubmed/26121203.
Rainkie D, Ensom MHH, Carr R. Pediatric assessment of vancomycin empiric dosing (PAVED): a retrospective review. pediatric drugs [Internet]. Springer International Publishing; 2015;17:245–53. https://dx.doi.org/10.1007/s40272-015-0122-8.
Seixas GTF, Araujo OR, Silva DCB, Arduini RG, Petrilli AS. Vancomycin therapeutic targets and nephrotoxicity in critically ill children with cancer. J Pediatr Hematol/Oncol [Internet]. 2016;38:e56–62. http://www.ncbi.nlm.nih.gov/pubmed/26558810.
da Silva DC, Seixas GT, Araujo OR, Arduini RG, Carlesse FA, Petrilli AS. Vancomycin serum concentrations in pediatric oncologic/hematologic intensive care patients. Braz J Infect Dis. 2012;16:361–5.
Zhang H, Wang Y, Gao P, Hu J, Chen Y, Zhang L, et al. Pharmacokinetic characteristics and clinical outcomes of vancomycin in young children with various degrees of renal function. J Clin Pharmacol. 2016;56:740–8.
De Cock PAJG, Desmet S, De Jaeger A, Biarent D, Dhont E, Herck I, et al. Impact of vancomycin protein binding on target attainment in critically ill children: back to the drawing board? J Antimicrob Chemother [Internet]. 2016;dkw495. https://academic.oup.com/jac/article-lookup/doi/10.1093/jac/dkw495.
Kishk OA, Lardieri AB, Heil EL, Morgan JA. Vancomycin AUC/MIC and corresponding troughs in a pediatric population. J Pediatr Pharmacol Therapeut [Internet]. 2017;22:41–7. https://www.jppt.org/doi/10.5863/1551-6776-22.1.41.
Thakkar N, Salerno S, Hornik CP, Gonzalez D. Clinical pharmacology studies in critically ill children. Pharmaceut Res [Internet]. 2016;1–18. https://dx.doi.org/10.1007/s11095-016-2033-y.
Funding
No sources of funding were used to conduct this study or prepare this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
ST and KC have no conflicts of interest. MHHE has held grants (not related to the article) from Medisca Pharmaceutique, Inc. in the past 36 months.
Rights and permissions
About this article
Cite this article
Tkachuk, S., Collins, K. & Ensom, M.H.H. The Relationship Between Vancomycin Trough Concentrations and AUC/MIC Ratios in Pediatric Patients: A Qualitative Systematic Review. Pediatr Drugs 20, 153–164 (2018). https://doi.org/10.1007/s40272-018-0282-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-018-0282-4