Abstract
Objectives
Our objectives were to explore the possibility of avoiding neonatal exposure to potentially harmful excipients of interest (EOI)—parabens, polysorbate 80, propylene glycol, benzoates, saccharin sodium, sorbitol and ethanol—through product substitution in Europe.
Methods
We performed a 3-day service evaluation survey and a 1-day point prevalence study in 20 and 21 European countries, respectively. Analysis included active pharmaceutical ingredients (APIs) used in ≥10 % of units. We calculated the potential reduction in number of products with EOI through substitution in three stages: (1) similar API and route of administration, (2) plus similar dosage form and (3) plus similar strength. The reduction of individual exposure was analysed according to the second-stage criteria.
Results
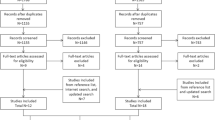
We identified 137 products for 25 APIs that contained EOI. Substitution with EOI-free product(s) was available for 88 % (n = 120), 66 % (n = 91) and 31 % (n = 42) of products according to the first-, second- and third-stage criteria, respectively. Overall, 456 (63 % of 726) neonates received products containing EOI. Substitution of the products that had alternatives with similar API and dosage form would reduce the number of exposed neonates from 456 to 257 (44 % reduction).
Conclusions
EOI-free formulations are available for a substantial number of products currently used in European neonates. Replacement of only the most frequently used products may spare almost half of neonates from unnecessary exposure to EOI.




Similar content being viewed by others
References
Fabiano V, Mameli C, Zuccotti GV. Paediatric pharmacology: remember the excipients. Pharmacol Res. 2011;63:362–5.
Rowe CR, Sheskey JP, Cook GW, Fenton EM. Handbook of pharmaceutical excipients. 7th ed. London: Pharmaceutical Press and American Pharmacists Association; 2012.
European Medicines Agency. Reflection paper: formulations of choice for the paediatric population. 2006; Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003782.pdf. Accessed 15 Mar 2016
Leeder JS, Kearns GL, Spielberg SP, van den Anker J. Understanding the relative roles of pharmacogenetics and ontogeny in pediatric drug development and regulatory science. J Clin Pharmacol. 2010;50:1377–87.
European Medicines Agency. Guideline on the investigation of medicinal products in the term and preterm neonate. 2009; http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003750.pdf. Accessed 15 Mar 2016
Hiller JL, Benda GI, Rahatzad M, Allen JR, David H, Carlson CV, et al. Benzyl alcohol toxicity: impact on mortality and intraventricular hemorrhage among very low birth weight infants. Pediatrics. 1986;77:500–6.
LeBel M, Ferron L, Masson M, Pichette J, Carrier C. Benzyl alcohol metabolism and elimination in neonates. Dev Pharmacol Ther. 1988;11:347–56.
Centers for Disease Control and Prevention. Neonatal deaths associated with use of benzyl alcohol. Morb Mortal Wkly Rep. 1982; http://www.cdc.gov/mmwr/preview/mmwrhtml/00001109.htm. Accessed 15 Mar 2016
Food and Drug Administration. Drug Safety and Availability—FDA Drug Safety Communication: serious health problems seen in premature babies given Kaletra (lopinavir/ritonavir) oral solution. Center for Drug Evaluation and Research. 2011; shttp://www.fda.gov/Drugs/DrugSafety/ucm246002.htm. Accessed 15 Mar 2016
European Medicines Agency. Questions and answers on generic medicines what is a generic medicine? 2012; http://www.ema.europa.eu/docs/en_GB/document_library/Medicine_QA/2009/11/WC500012382.pdf. Accessed 15 Mar 2016
Turner M. European study of neonatal exposure to excipients (ESNEE). Infant. 2011;7:1–4.
Turner MA, Duncan J, Shah U, Metsvaht T, Varendi H, Nellis G, et al. European study of neonatal exposure to excipients: an update. Int J Pharm. 2013;457:357–8.
Nellis G, Metsvaht T, Varendi H, Toompere K, Lass J, Mesek I, et al. Potentially harmful excipients in neonatal medicines: a pan-European observational study. Arch Dis Child. 2015;100:694–9.
Lass J, Naelapää K, Shah U, Käär R, Varendi H, Turner MA, et al. Hospitalised neonates in Estonia commonly receive potentially harmful excipients. BMC Pediatr. 2012;12:136.
Souza A, Dos Santos DB, Fonseca S, Medeiros M, Batista L, Turner M, et al. Toxic excipients in medications for neonates in Brazil. Eur J Pediatr. 2014;173(7):935–45.
de Souza AS, dos Santos DB, Rey LC, Medeiros MG, Vieira MG, Coelho HLL. Off-label use and harmful potential of drugs in a NICU in Brazil: a descriptive study. BMC Pediatr. 2016;16:13.
Turner MA, Duncan JC, Shah U, Metsvaht T, Varendi H, Nellis G, et al. Risk assessment of neonatal excipient exposure: lessons from food safety and other areas. Adv Drug Deliv Rev. 2014;73C:89–101.
Nellis G, Lutsar I, Varendi H, Toompere K, Turner MA, Duncan J, et al. Comparison of two alternative study designs in assessment of medicines utilisation in neonates. BMC Med Res Methodol. 2014;14:89.
Fortescue EB, Kaushal R, Landrigan CP, Mckenna KJ, Clapp MD, Federico F, et al. Prioritizing strategies for preventing medication errors and adverse drug events in pediatric inpatients. Pediatrics. 2003;111:722–9.
Chedoe I, Molendijk HA, Dittrich STAM, Jansman FGA, Harting JW, Brouwers JRBJ. Incidence and nature of medication errors in neonatal intensive care with strategies to improve safety. Drug Saf. 2007;30:503–13.
European Medicines Agency. Guideline on pharmaceutical development of medicines for paediatric use. 2013; http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/07/WC500147002.pdf. Accessed 15 Mar 2016
Ivanovska V, Rademaker CMA, van Dijk L, Mantel-Teeuwisse AK. Pediatric drug formulations: a review of challenges and progress. Pediatrics. 2014;134:361–72.
Turner MA. Neonatal drug development. Early Hum Dev. 2011;87:763–8.
Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857–72.
Lowry R. VassarStats: website for statistical computation. http://vassarstats.net/. Accessed 15 Mar 2016
ISO 3166—Country codes. http://www.iso.org/iso/home/standards/country_codes.htm. Accessed 15 Mar 2016
European Medicines Agency. Questions and answers on benzoic acid and benzoates in the context of the revision of the guideline on “Excipients in the label and package leaflet of medicinal products for human use” (CPMP/463/00). 2014; http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/02/WC500162031.pdf. Accessed 15 Mar 2016
European Medicines Agency. Questions & answers on propylene glycol and esters in the context of the revision of the guideline on “Excipients in the label and package leaflet of medicinal products for human use” (CPMP/463/00). 2014; http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/12/WC500177945.pdf. Accessed 15 Mar 2016
Whittaker A, Currie A, Turner MA, Field DJ, Mulla H, Pandya HC. Toxic additives in medication for preterm infants. Arch Dis Child Fetal Neonatal Ed. 2009;94:236–40.
Shehab N, Lewis CL, Streetman DD, Donn SM. Exposure to the pharmaceutical excipients benzyl alcohol and propylene glycol among critically ill neonates. Pediatr Crit Care Med. 2009;10:256–9.
Simoens S. Pricing and reimbursement of orphan drugs: the need for more transparency. Orphanet J Rare Dis. 2011;6:42.
European Medicines Agency. 5-year Report to the European Commission: general report on the experience acquired as a result of the application of the Paediatric Regulation. 2012; http://ec.europa.eu/health/files/paediatrics/2012-09_pediatric_report-annex1-2_en.pdf. Accessed 15 Mar 2016
Mason J, Pirmohamed M, Nunn T. Off-label and unlicensed medicine use and adverse drug reactions in children: a narrative review of the literature. Eur J Clin Pharmacol. 2012;68:21–8.
Lass J, Käär R, Jõgi K, Varendi H, Metsvaht T, Lutsar I. Drug utilisation pattern and off-label use of medicines in Estonian neonatal units. Eur J Clin Pharmacol. 2011;67:1263–71.
Mulla H, Yakkundi S, McElnay J, Lutsar I, Metsvaht T, Varendi H, et al. An observational study of blood concentrations and kinetics of methyl- and propyl-parabens in neonates. Pharm Res. 2015;32(3):1084–93.
Salunke S, Brandys B, Giacoia G, Tuleu C. The STEP (Safety and Toxicity of Excipients for Paediatrics) database: part 2—the pilot version. Int J Pharm. 2013;457:310–22.
Acknowledgments
Members of ESNEE are as follows: Susan Graham (UK), Utpal Shah (UK), Hussain Mulla (UK), Hitesh Pandya (UK), James McElnay (UK), Jeff Millership (UK), Shirish Yakkundi (UK), Andre Rieutord (France), Thomas Storme (France), Pascal Vaconsin (France). All members of ESNEE designed the study and monitored data collection. The authors thank all national contact people who provided lists of neonatal units and helped run the study in their own country: Bernhard Resch (Austria), Pieter De Cock (Belgium), Nelly Jekova (Bulgaria), Elisabeth Iyore (Denmark), Pascal Vaconsin (France), Kosmas Sarafidis (Greece), Aranka Vegso (Hungary), Noreen O’Callaghan (Ireland), Rocco Agostino (Italy), Daiga Kviluna (Latvia), Rasa Tameliene (Lithuania), Rene F. Kornelisse (Netherlands), Dag Bratlid (Norway), Almerinda Pereira (Portugal), Maria Livia Ognean (Romania), Milica Bajcetic (Serbia), Darja Paro (Slovenia), Elizabeth Valls (Spain), Per Nydert (Sweden), Hans Ulrich Bucher (Switzerland), Maria Cordina (Malta). We also thank local pharmacists for providing data on excipient content: Caroline Fonzo-Christe (Switzerland), Domenico Tarantino (Italy), Velina Grigorova (Bulgaria), Milica Bajcetic (Serbia), Elizabeth Valss (Spain), Claudine Milstein (France), Jennifer Duncan (England), Sabina Zalar (Slovenia), Per Gustaf Hartvig Honoré (Denmark).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
GN, TM, HV, JL, JD, AJN, MAT and IL have completed the conflict of interest disclosure form(s) and declare they received no support from any organisation for the submitted work and have no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years. JL, AJN and IL are members of the Paediatric Committee (PDCO) of the European Medicines Agency; MAT is a Chair of the European Network for Paediatric Research at the European Medicines Agency (EnprEMA); AJN is an independent scientific expert to the European Medicines Agency and a member of the working group revising the EU guideline “Excipients in the Label and Package Leaflet of Medicinal Products for Human Use”.
Funding
ESNEE is funded through European Research Area-Network PRIOMEDCHILD by the following national agencies: Medical Research Council from the UK, Estonian Research Council (IUT 34-24) from Estonia, Agence Nationale de la Recherche from France. The funder of the study had no role in study design, data collection, data analysis, data interpretation, writing of the report, or in the decision to submit the paper for publication.
Transparency Declaration
The corresponding author affirms this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained. The corresponding author confirms he had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Ethical Considerations
The SES was performed as part of an audit with no personal data collected, and no Ethics Committee approval was required in participating countries. For the PPS, Ethics Committee approval was obtained in compliance with national guidelines. All data were anonymised before leaving the study sites.
Data sharing
No additional data available.
Additional information
On behalf of the ESNEE consortium.
Rights and permissions
About this article
Cite this article
Nellis, G., Metsvaht, T., Varendi, H. et al. Product Substitution as a Way Forward in Avoiding Potentially Harmful Excipients in Neonates. Pediatr Drugs 18, 221–230 (2016). https://doi.org/10.1007/s40272-016-0173-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-016-0173-5