Abstract
Background
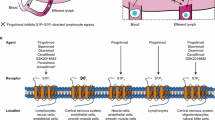
Several sphingosine-1-phosphate receptor (S1PR) modulators are available in the US for treating relapsing forms of multiple sclerosis (RMS). Given that these S1PR modulators have similar efficacy and safety, patients may consider the clinical management characteristics of the S1PR modulators when deciding among treatments. However, none of the S1PR modulators is clearly superior in every aspect of clinical management, and for some treatments, clinical management varies based on a patient’s comorbid health conditions (e.g., heart conditions [HC]).
Objectives
This study aimed to determine which S1PR modulator patients with relapsing-remitting multiple sclerosis (RRMS) would prefer based on clinical management considerations, and to estimate how different clinical management considerations might drive these preferences. Preferences were explored separately for patients with and without comorbid HC.
Methods
A multicriteria decision analysis was conducted on S1PR modulators approved to treat RMS: fingolimod, ozanimod, siponimod, and ponesimod. Clinical management preferences of patients with RRMS were elicited in a discrete choice experiment (DCE) in which participants repeatedly chose between hypothetical S1PR modulator profiles based on their clinical management attributes. Attributes included first-dose observations, genotyping, liver function tests, eye examinations, drug–drug interactions, interactions with antidepressants, interactions with foods high in tyramine, and immune system recovery time. Preferences were estimated separately for patients with HC and without HC (noHC). Marginal utilities were calculated from the DCE data for each attribute and level using a mixed logit model. In the multicriteria decision analysis, partial value scores were created by applying the marginal utilities for each attribute and level to the real-world profiles of S1PR modulators. Partial value scores were summed to determine an overall clinical management value score for each S1PR modulator.
Results
Four hundred patients with RRMS completed the DCE. Ponesimod had the highest overall value score for patients both without (n = 341) and with (n = 59) HC (noHC: 5.1; HC: 4.0), followed by siponimod (noHC: 4.9; HC: 3.3), fingolimod (noHC: 3.4; HC: 2.8), and ozanimod (noHC: 0.9; HC: 0.8). Overall, immune system recovery time contributed the highest partial value scores (noHC: up to 1.9 points; HC: up to 1.2 points), followed by the number of drug–drug interactions (noHC: up to 1.2 points; HC: up to 1.7 points).
Conclusions
When considering the clinical management of S1PR modulators, the average patient with RRMS is expected to choose a treatment with shorter immune system recovery time and fewer interactions with other drugs. Patients both with and without heart conditions are likely to prefer the clinical management profile of ponesimod over those of siponimod, fingolimod, and ozanimod. This information can help inform recommendations for treating RRMS and facilitate shared decision making between patients and their doctors.



Similar content being viewed by others
References
Calabresi PA. Diagnosis and management of multiple sclerosis. Am Fam Phys. 2004;70(10):1935–44.
Goldenberg MM. Multiple sclerosis review. P T. 2012;37(3):175–84.
Browne P, Chandraratna D, Angood C, Tremlett H, Baker C, Taylor BV, Thompson AJ. Atlas of multiple sclerosis 2013: a growing global problem with widespread inequity. Neurology. 2014;83(11):1022–4.
GBD 2016 Motor Neuron Disease Collaborators. Global, regional, and national burden of motor neuron diseases 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(12):1083-97.
Crayton H, Heyman RA, Rossman HS. A multimodal approach to managing the symptoms of multiple sclerosis. Neurology. 2004;63(11 Suppl 5):S12–8.
Crayton HJ, Rossman HS. Managing the symptoms of multiple sclerosis: a multimodal approach. Clin Ther. 2006;28(4):445–60.
Kappos L, Radue E-W, O’Connor P, Polman C, Hohlfeld R, Calabresi P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362(5):387–401.
Chun J, Giovannoni G, Hunter SF. Sphingosine 1-phosphate receptor modulator therapy for multiple sclerosis: differential downstream receptor signalling and clinical profile effects. Drugs. 2021;81(2):207–31.
Novartis Pharmaceuticals Corporation. GILENYA (fingolimod) capsules [package insert]. US FDA; 2012.
Novartis Pharmaceuticals Corporation. MAYZENT® (siponimod) tablets, for oral use [package insert]. US FDA; 2019.
Celgene Corporation. ZEPOSIA® (ozanimod) capsules, for oral use [package insert]. US FDA; 2020.
Janssen Pharmaceuticals. PONVORY™ (ponesimod) tablets, for oral use [package insert]. US FDA; 2021.
Hennessy B, Zierhut ML, Kracker H, Keenan A, Sidorenko T. Comparative efficacy of relapsing multiple sclerosis therapies: model-based meta-analyses for confirmed disability accumulation and annualized relapse rate. Mult Scler Relat Disord. 2022;64: 103908.
Tong J, Zou Q, Chen Y, Liao X, Chen R, Zhang D, Li Q. Efficacy and acceptability of the S1P receptor in the treatment of multiple sclerosis: a meta-analysis. Neurol. Sci. 2021;42.
Belton V, Stewart T. Multiple criteria decision analysis: an integrated approach. New York: Springer; 2002.
Clark MD, Determann D, Petrou S, Moro D, de Bekker-Grob EW. Discrete choice experiments in health economics: a review of the literature. Pharmacoeconomics. 2014;32(9):883–902.
Soekhai V, de Bekker-Grob EW, Ellis AR, Vass CM. Discrete choice experiments in health economics: past, present and future. Pharmacoeconomics. 2019;37(2):201–26.
Ryan M, Bate A, Eastmond CJ, Ludbrook A. Use of discrete choice experiments to elicit preferences. Qual Health Care. 2001;10(Suppl 1):i55–60.
Patten SB. Current perspectives on co-morbid depression and multiple sclerosis. Expert Rev Neurother. 2020;20(8):867–74.
Patten SB, Marrie RA, Carta MG. Depression in multiple sclerosis. Int Rev Psychiatry. 2017;29(5):463–72.
Siegert RJ, Abernethy DA. Depression in multiple sclerosis: a review. J Neurol Neurosurg Psychiatry. 2005;76(4):469.
Campoamor NB, Guerrini CJ, Brooks WB, Bridges JFP, Crossnohere NL. Pretesting discrete-choice experiments: a guide for researchers. The Patient Patient-Center Outcomes Res. 2024;17(2):109–20.
Reed Johnson F, Lancsar E, Marshall D, Kilambi V, Muhlbacher A, Regier DA, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health. 2013;16(1):3–13.
de Bekker-Grob EW, Donkers B, Jonker MF, Stolk EA. Sample size requirements for discrete-choice experiments in healthcare: a practical guide. Patient. 2015;8(5):373–84.
Rose J, Bliemer M. Sample size requirements for stated choice experiments. Transportation. 2013;40.
ChoiceMetrics. Ngene 1.2 User Manual and Reference Guide 2019.
Rose JM, Bliemer MC. Constructing efficient stated choice experimental designs. Transp Rev. 2009;29(5):587–617.
Johnson FR, Yang JC, Reed SD. The internal validity of discrete choice experiment data: a testing tool for quantitative assessments. Value Health. 2019;22(2):157–60.
Lancsar E, Louviere J. Deleting “irrational” responses from discrete choice experiments: a case of investigating or imposing preferences? Health Econ. 2006;15(8):797–811.
Hauber AB, González JM, Groothuis-Oudshoorn CGM, Prior T, Marshall DA, Cunningham C, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR conjoint analysis good research practices task force. Value Health. 2016;19(4):300–15.
McFadden D. Econometric models of probabilistic choice. In: Manski C, McFadden D, editors. Structural analysis of discrete data with econometric applications. Cambridge: MIT Press; 1981.
Lancsar E, Fiebig DG, Hole AR. Discrete choice experiments: a guide to model specification, estimation and software. Pharmacoeconomics. 2017;35(7):697–716.
Jonker MF, Donkers B, Goossens LMA, Hoefman RJ, Jabbarian LJ, de Bekker-Grob EW, et al. Summarizing patient preferences for the competitive landscape of multiple sclerosis treatment options. Med Decis Making. 2020;40(2):198–211.
Webb EJD, Meads D, Eskyte I, King N, Dracup N, Chataway J, et al. A systematic review of discrete-choice experiments and conjoint analysis studies in people with multiple sclerosis. Patient. 2018;11(4):391–402.
Alonso R, Carnero Contentti E, Graña M, Linares R, Lopez P, Mainella C, et al. Shared decision making in the treatment of multiple sclerosis: a consensus based on Delphi methodology. Multiple Scler Relat Disord. 2023;70(104465).
Ubbink DT, Damman OC, de Jong BA. Shared decision-making in patients with multiple sclerosis. Front Neurol. 2022;13:1063904.
Bridges JF, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, et al. Conjoint analysis applications in health—a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14(4):403–13.
International Society for Pharmacoeconomics Outcomes Research (ISPOR). ISPOR Good Practices for Outcomes Research Index 2018. https://www.ispor.org/heor-resources/good-practices-for-outcomes-research.
de Bekker-Grob EW, Swait JD, Kassahun HT, Bliemer MCJ, Jonker MF, Veldwijk J, et al. Are healthcare choices predictable? The impact of discrete choice experiment designs and models. Value Health. 2019;22(9):1050–62.
Quaife M, Terris-Prestholt F, Di Tanna GL, Vickerman P. How well do discrete choice experiments predict health choices? A systematic review and meta-analysis of external validity. Eur J Health Econ. 2018;19(8):1053–66.
Acknowledgements
Medical writing was provided by Jacqueline Janowich Wasserott, PhD (Evidera), and funded by Janssen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Janssen. The sponsor was involved in the study design but had no role in data collection, data analysis, data interpretation, or writing of the statistical report. All authors had full access to the study data and had final responsibility for the decision to submit this article for publication. CW, GSF, VT, AD, and MQ are employees of Evidera, part of the clinical research group within Thermo Fisher Scientific, which received funding from Janssen to conduct this study. AK, HHL, and DMK are employees of Janssen and may own Johnson & Johnson stocks. APR reports having received consulting fees from Janssen. Medical writing was provided by Jacqueline Janowich Wasserott (Evidera) and was funded by Janssen.
Author contributions
AK: Conceptualization, methodology, software, writing—original draft, writing—review and editing, supervision. CW: Conceptualization, methodology, validation, formal analysis, investigation, data curation, writing—original draft, writing—review and editing, visualization, supervision, project administration. HHL: Conceptualization, methodology, writing—original draft, writing—review and editing, supervision. DMK: Conceptualization, methodology, writing—review and editing, supervision. GSF: Conceptualization, methodology, validation, investigation, data curation, writing—original draft, writing—review and editing, project administration. VT: Methodology, validation, investigation, data curation, writing—original draft, writing—review and editing. AD: Methodology, validation, formal analysis, investigation, data curation, writing—review and editing, visualization. MQ: Conceptualization, methodology, validation, formal analysis, investigation, data curation, writing—original draft, writing—review and editing, supervision. APR: Conceptualization, writing—review and editing.
Conflict of Interest
Chiara Whichello, Gabriela S. Fernandez, Vicky Turner, Anup Das, and Matthew Quaife are employees of Evidera, which received funding from Janssen to conduct this study. Alexander Keenan, Hoa H. Le, and David M. Kern are employees of Janssen and may own Johnson & Johnson stocks. Amy Perrin Ross reports having received consulting fees from EMD Serono, BMS, Novartis, Janssen, Genzyme, Horizon, Alexion, TG Therapeutics, Roche, and Merke, as well as payments or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from EMD Serono, BMS, Novartis, Genzyme, Horizon, Alexion, and TG Therapeutics.
Data Availability
The informed consent obtained from participants does not allow for individual data to be made publicly available.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Keenan, A., Whichello, C., Le, H.H. et al. Patients’ Preferences for Sphingosine-1-Phosphate Receptor Modulators in Multiple Sclerosis Based on Clinical Management Considerations: A Choice Experiment. Patient (2024). https://doi.org/10.1007/s40271-024-00699-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s40271-024-00699-2