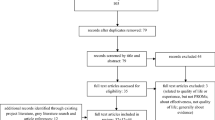
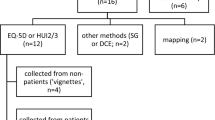
Abstract
Rare diseases are often severe, debilitating, life-limiting conditions, many of which occur in childhood. These complex conditions have a wide range of clinical manifestations that have a substantial impact on the lives of patients, carers and families and often produce heterogeneous clinical outcomes. Therefore, the evaluation of quality-of-life (QoL) impacts is important. In health technology assessment (HTA), patient-reported outcome measures (PROMs) and/or health state utility values (HSUVs) are used to determine QoL impacts of new treatments, but their use in rare diseases is challenging due to small and heterogeneous populations and limited disease knowledge. This paper describes challenges associated with the use of patient-reported outcomes (PROs)/HSUVs to evaluate QoL in HTA of rare disease treatments (RDTs) and identifies five recommendations to ensure appropriate interpretation of QoL impacts. These were derived from mixed methods research (literature reviews, appraisal document analyses, appraisal committee observations and interviews) examining the use of PROs/HSUVs in HTA of RDTs. They highlight that HTAs of RDTs must (1) understand the QoL impacts of the disease and of treatments; (2) critically assess PRO data, recognising the nuances in development and administration of PROMs/HSUVs, considering what is feasible and what matters most to the patient population; (3) recognise that lack of significant effect on a PRO does not imply no QoL benefit; (4) use different forms of evidence to understand QoL impacts, such as patient input; and (5) provide methodological guidance to capture QoL impacts on patients/carers.
Similar content being viewed by others
References
FDA. Orphan Drug Act [Internet]. Available from: https://www.fda.gov/industry/designating-orphan-product-drugs-and-biological-products/e-cfr-21-part-316-orphan-drug-orphan-drug-act-1983
Regulation (EC) No 141/2000 of the European Parliament and of the Council of 16 December 1999 on orphan medicinal products. Off J Eur Union. 2000;
European Commission. Orphan medicinal products [Internet]. [cited 2022 Jan 7]. Available from: https://ec.europa.eu/health/human-use/orphan-medicines_en
Eurordis. About rare diseases [Internet]. [cited 2021 Mar 10]. Available from: https://www.eurordis.org/about-rare-diseases
Drummond M, Sculpher M, Claxton K, Stoddart G, Torrance G. Measuring and valuing health effects. Methods Econ Eval Heal care. Fourth. Oxford: Oxford University Press; 2015. p. 123–80.
Hong YD, Villalonga-Olives E, Perfetto EM. Patient-Reported Outcomes in Orphan Drug Labels Approved by the US Food and Drug Administration. Value Heal [Internet]. 2019;22:925–30. Available from: https://www.sciencedirect.com/science/article/pii/S1098301519301834
Longworth L, Rowen D. Mapping to obtain EQ-5D utility values for use in nice health technology assessments. Value Heal. 2013;16:202–10.
Brazier J, Rowen D. NICE DSU technical support document 11: alternatives to EQ5D for generating health state utility values. 2011.
Nicod E, Annemans L, Bucsics A, Lee A, Upadhyaya S, Facey K. HTA programme response to the challenges of dealing with orphan medicinal products: process evaluation in selected European countries. Health Policy (New York). 2019;123:140–51.
Nguengang Wakap S, Lambert DM, Olry A, Rodwell C, Gueydan C, Lanneau V, et al. Estimating cumulative point prevalence of rare diseases: analysis of the Orphanet database. Eur J Hum Genet. 2020;
Devlin NJ. Valuing Child Health Isn’t Child’s Play. Value Heal. 2022.
National Institute for Health and Care Excellence. NICE health technology evaluations: the manual [Internet]. Process methods. [cited 2022 Jun 13]. Available from: www.nice.org.uk/process/pmg36
Luzzatto L, Hyry H, Schieppati A, Costa E, Simoens S, Schaefer F. Outrageous prices of orphan drugs: a call for collaboration. Lancet. 2018;392:791–4.
Nicod E, Maynou L, Maynou L, Visintin E, Cairns J. Why do health technology assessment drug reimbursement recommendations differ between countries? A parallel convergent mixed methods study. Heal Econ Policy Law. 2020;15.
Nicod E, Meregaglia M, Whittal A, Upadhyaya S, Facey K, Drummond M, et al. Consideration of health-related quality of life in the health technology assessment of rare disease treatments. Eur J Heal Econ. 2021;
Hill H, Rowen D, Pennington B, Wong R, Wailoo A. A review of the methods used to generate utility values in NICE technology assessments for children and adolescents. Value Heal. 2020;23:907–17.
Matza LS, Stewart KD, Lloyd AJ, Rowen D, Brazier JE. Vignette-Based Utilities: Usefulness, Limitations, and Methodological Recommendations. Value Heal J Int Soc Pharmacoeconomics Outcomes Res. United States; 2021;24:812–21.
Meregaglia M, Nicod E, Drummond M. The estimation of health state utility values in rare diseases: overview of existing techniques. Int J Technol Assess Health Care. 2020;36:469–73.
Thompson R, Vaidya S, Teynor M. The Utility of Different Approaches to Developing Health Utilities Data in Childhood Rare Diseases – A Case Study in Spinal Muscular Atrophy. Value Heal. 2017;A399–811.
Nicod E, Meragaglia M, Whittal A, Upadhyaya S, Facey K, Drummond M, et al. Consideration of quality of life in the health technology assessment of rare disease treatments. Eur J Heal Econ. 2022;23:645–69.
Whittal A, Meragaglia M, Nicod E. The use of patient-reported outcome measures in rare diseases and implications for health technology assessment. Patient. 2021;14:485–503.
Meregaglia M, Whittal A, Nicod E, Drummond M. ‘Mapping’ health state utility values from non-preference-based measures: a systematic literature review in rare diseases. Pharmacoeconomics. 2020;38:557–74.
Whittal A, Nicod E, Drummond M, Facey K. Country vignettes on HTA appraisal/reimbursement processes for rare disease treatments [Internet]. 2020 [cited 2020 Feb 26]. Available from: https://www.impact-hta.eu/country-vignettes
Facey K, Whittal A, Drummond M, Upadhyaya S, Junghans T, Nicod E. WP10 HTA Appraisal Framework Suitable for Rare Disease Treatments [Internet]. 2021. Available from: https://8c3e11d9-5f36-452f-abe3-c95befd6e85d.filesusr.com/ugd/e1a359_bdba35aaa891440b8475cf174b937407.pdf
Bogart KR, Irvin VL. Health-related quality of life among adults with diverse rare disorders. Orphanet J Rare Dis. 2017;12:177.
Bryson B, Bogart K, Atwood M, Fraser K, Locke T, Pugh K, et al. Navigating the unknown: A content analysis of the unique challenges faced by adults with rare diseases. J Health Psychol [Internet]. SAGE Publications Ltd; 2019;1359105319828150. Available from: https://doi.org/10.1177/1359105319828150
Lenderking W, Anatchkova M, Pokrzywinski R, Skalicky A, Martin M, Gelhorn H. Measuring health-related quality of life in patients with rare disease. J Patient-Reported Outcomes. 2021;5.
Nicod E, Whittal A, Drummond M, Facey K. Are supplemental appraisal/reimbursement processes needed for rare disease treatments? An international comparison of country approaches. Orphanet J Rare Dis. Orphanet Journal of Rare Diseases; 2020;15:1–14.
Morel T, Cano SJ. Measuring what matters to rare disease patients—reflections on the work by the IRDiRC taskforce on patient-centered outcome measures. Orphanet J Rare Dis. 2017;12:171.
Benjamin K, Vernon MK, Patrick DL, Perfetto E, Nestler-Parr S, Burke L. Patient-reported outcome and observer-reported outcome assessment in rare disease clinical trials: an ISPOR COA emerging good practices task force report. Value Heal. 2017;20:838–55.
Tosi LL, Floor MK, Dollar CM, Gillies AP, Lee B, Nagamani SCS, et al. Assessing disease experience across the life span for individuals with osteogenesis imperfecta: challenges and opportunities for patient-reported outcomes (PROs) measurement: a pilot study. Orphanet J Rare Dis. 2019;14:23.
Whitehurst DGT, Suryaprakash N, Engel L, Mittmann N, Noonan VK, Dvorak MFS, et al. Perceptions of individuals living with spinal cord injury toward preference-based quality of life instruments: a qualitative exploration. Health Qual Life Outcomes. 2014;12:50.
Slade A, Isa F, Kyte D, Pankhurst T, Kerecuk L, Ferguson J, et al. Patient reported outcome measures in rare diseases: a narrative review. Orphanet J Rare Dis. 2018;13:61.
Hartley T, Lemire G, Kernohan KD, Howley HE, Adams DR, Boycott KM. New Diagnostic Approaches for undiagnosed rare genetic diseases. Annu Rev Genomics Hum Genet [Internet]. Annual Reviews; 2020;21:351–72. Available from: https://doi.org/10.1146/annurev-genom-083118-015345
Powell PA, Carlton J, Rowen D, Chandler F, Guglieri M, Brazier JE. Development of a new quality of life measure for duchenne muscular dystrophy using mixed methods: the DMD-QoL. Neurology. 2021;96:e2438–50.
Bell JA, Galaznik A, Pompilus F, Strzok S, Bejar R, Scipione F, et al. A pragmatic patient-reported outcome strategy for rare disease clinical trials: application of the EORTC item library to myelodysplastic syndromes, chronic myelomonocytic leukemia, and acute myeloid leukemia. J Patient-Reported Outcomes. 2019;3:35.
Pascoal C, Brasil S, Francisco R, Marques-Da-Silva D, Rafalko A, Jaeken J, et al. Patient and observer reported outcome measures to evaluate health-related quality of life in inherited metabolic diseases: a scoping review. Orphanet J Rare Dis. 2018;13:215.
Mack JW, McFatrich M, Withycombe JS, Maurer SH, Jacobs SS, Lin L, et al. Agreement between child self-report and caregiver-proxy report for symptoms and functioning of children undergoing cancer treatment. JAMA Pediatr. 2020;174: e202861.
Burström K, Bartonek Å, Broström EW, Sun S, Egmar A-C. EQ-5D-Y as a health-related quality of life measure in children and adolescents with functional disability in Sweden : testing feasibility andvalidity. Acta Paediatr [Internet]. The Swedish Red Cross University College: John Wiley & Sons; 2014;103:426–35. Available from: http://urn.kb.se/resolve?urn=urn:nbn:se:rkh:diva-836
National Institute for Health and Care Excellence. Afamelanotide for treating erythropoietic protoporphyria [ID927] [Internet]. 2022 [cited 2022 Jun 28]. Available from: https://www.nice.org.uk/guidance/indevelopment/gid-hst10009
Mendell JR, Al-Zaidy S, Shell R, Arnold WD, Rodino-Klapac LR, Prior TW, et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377:1713–22.
Basch E, Bennett A V. Patient-Reported Outcomes in Clinical Trials of Rare Diseases. J Gen Intern Med [Internet]. 2014;29:801–3. Available from: https://doi.org/10.1007/s11606-014-2892-z
Swezey T, Reeve BB, Hart TS, Floor MK, Dollar CM, Gillies AP, et al. Incorporating the patient perspective in the study of rare bone disease: insights from the osteogenesis imperfecta community. Osteoporos Int. 2019;30:507–11.
Bray N, Noyes J, Harris N, Edwards RT. Measuring the health-related quality of life of children with impaired mobility: examining correlation and agreement between children and parent proxies. BMC Res Notes. 2017;10:377.
Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. Patient-focused drug development: collecting comprehensive and representative input. Guidance for industry, food and drug administration staff, and other stakeholders. 2020; Available from: https://www.fda.gov/media/139088/download
ICH Reflection paper. Proposed ICH Guideline Work to Advance Patient Focused Drug Development [Internet]. 2020. Available from: https://admin.ich.org/sites/default/files/2020-12/ICH_ReflectionPaper_PFDD_Endorsed-ForConsultation_2020_1118.pdf
Calvert M, Kyte D, Mercieca-Bebber R, Slade A, Chan A-W, King MT, et al. Guidelines for Inclusion of Patient-Reported Outcomes in Clinical Trial Protocols: The SPIRIT-PRO Extension. JAMA [Internet]. 2018;319:483–94. Available from: https://doi.org/10.1001/jama.2017.21903
Rüther A, Elstein D, Wong-Rieger D, Guyatt G. Aspects of patient reported outcomes in rare diseases: a discussion paper. Int J Technol Assess Health Care. 2016;32:126–30.
Lanar S, Acquadro C, Seaton J, Savre I, Arnould B. To what degree are orphan drugs patient-centered? A review of the current state of clinical research in rare diseases. Orphanet J Rare Dis [Internet]. 2020;15:134. Available from: https://doi.org/10.1186/s13023-020-01400-0
Towse A, Garau M. Appraising ultra-orphan drugs: is cost-per-QALY appropriate? A review of the evidence [Internet]. 2018. Available from: https://www.ohe.org/publications/appraising-ultra-orphan-drugs-cost-qaly-appropriate-review-evidence
Rowen D, Brazier J, Wong R, Wailoo A. Measuring and valuing health-related quality of life when sufficient EQ-5D data is not available [Internet]. Sheffield; 2020. Available from: http://nicedsu.org.uk/wp-content/uploads/2020/11/DSU-hierarchy-of-evidence-report-310720-Final-for-website.pdf
Lloyd A, Aggio D, Slocomb TL, Lee J, Beggs AH, Bilder DA. Estimation of the quality-of-life impact of x-linked myotubular myopathy. J Neuromuscul Dis. 2021;8:1047–61.
Pearson I, Rothwell B, Olaye A, Knight C. Economic modeling considerations for rare diseases. Value Heal. 2018;21:515–24.
Arnold D, Girling A, Stevens A, Lilford R. Comparison of direct and indirect methods of estimating health state utilities for resource allocation: review and empirical analysis. BMJ. 2009;339:2688.
NICE Guidance. Nusinersen for treating spinal muscular atrophy - TA588 [Internet]. Available from: https://www.nice.org.uk/guidance/ta588
NICE Guidance. Cerliponase alfa for treating neuronal ceroid lipofuscinosis type 2 [Internet]. 2019. Available from: https://www.nice.org.uk/guidance/hst12
Lloyd AJ, Thompson R, Gallop K, Teynor M. Estimation Of the quality of life benefits associated with treatment for spinal muscular atrophy. Clinicoecon Outcomes Res. 2019;11:615–22.
NICE Guidance. Volanesorsen for treating familial chylomicronaemia syndrome [Internet]. 2020. Available from: https://www.nice.org.uk/guidance/hst13
NICE Guidance. Voretigene neparvovec for treating inherited retinal dystrophies caused by RPE65 gene mutations [Internet]. 2019. Available from: https://www.nice.org.uk/guidance/hst11
Facey K, Ploug Hansen H, Single A. Patient input into HTA. Patient Involv Heal Technol Assess. 2017. p. 67–9.
Eurordis. Juggling care and daily life [Internet]. [cited 2021 Mar 16]. Available from: http://download2.eurordis.org/rbv/juggling_care_and_daily_life.infographic__final.pdf
Simpson A, Bloom L, Fulop NJ, Hudson E, Leeson-Beevers K, Morris S, et al. How are patients with rare diseases and their carers in the UK impacted by the way care is coordinated? An exploratory qualitative interview study [Internet]. Orphanet J. Rare Dis. Genetic Alliance UK, Third Floor, 86-90 Paul Street, London, EC2A 4NE, UK. amy.simpson@geneticalliance.org.uk.; 2021. p. 76. Available from: http://europepmc.org/abstract/MED/33568181
Pennington B, Wong R. Modelling carer health-related quality of life in NICE Technology Appraisals and Highly Specialised Technologies [Internet]. 2019 [cited 2022 Jun 28]. p. 49. Available from: https://drive.google.com/file/d/18ULRLvDy9-iMCnuEBs-RSuXVg5h64SCT/view
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Authors contributions
All authors made substantial contributions to the design of the work. EN drafted the work; all authors revised it critically at several occasions for important intellectual content; all authors approved the version being submitted. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This research was partially funded by the European Commission’s Horizon 2020 research and innovation programme and was undertaken under the auspices of IMPACT-HTA (Grant number 779312). The results presented here reflect the authors’ views and not the views of the European Commission. The European Commission is not liable for any use of the information communicated.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Data availability
All data generated or analysed during this study are referenced in this published article.
Code availability
Not applicable.
Competing interests
Outside of this research: Elena Nicod and Amanda Whittal are also employed by Dolon Ltd. Andrew Lloyd is an employee and shareholder in Acaster Lloyd Consulting Ltd, which receives fees from the pharmaceutical industry to undertake research projects. Michael Drummond has received fees from several pharmaceutical companies to provide advice on HTA. Karen Facey has received fees from several pharmaceutical companies to provide advice on HTA and patient involvement, and from public bodies to provide facilitation. Thomas Morel is an employee and shareholder of UCB Pharma. Sheela Upadhyaya and Michela Meregaglia do not have any competing interests.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nicod, E., Lloyd, A.J., Morel, T. et al. Improving Interpretation of Evidence Relating to Quality of Life in Health Technology Assessments of Rare Disease Treatments. Patient 16, 7–17 (2023). https://doi.org/10.1007/s40271-022-00598-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40271-022-00598-4