Abstract
Background
Few studies have examined pediatric rheumatologists’ approaches to treatment decision making for biologic therapy for patients with juvenile idiopathic arthritis (JIA). This study presents the qualitative research undertaken to support the development of a Best–Worst Scaling (BWS) survey for tapering in JIA. The study objectives were to (1) describe the treatment decision-making process of pediatric rheumatologists to initiate and taper biologics; and (2) select attributes for a BWS survey.
Methods
Pediatric rheumatologists across Canada were recruited to participate in interviews using purposeful sampling. Interviews were conducted until saturation was achieved. Interview recordings were transcribed verbatim and transcripts were analyzed using deductive thematic analysis. Initial codes were organized into themes and subthemes using an iterative process. Attributes for the BWS survey were developed from these themes and a literature review was conducted in parallel to inform survey development. Further refinement of the attributes was done through consultation with the research team.
Results
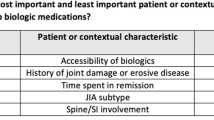
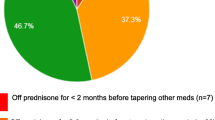
Five pediatric rheumatologists participated in the interviews. Shared decision making was part of the approach to initiating and tapering biologics in their practice. Tapering approaches differed; some pediatric rheumatologists preferred to stop biologics immediately, while others tapered by reducing dose and/or increasing the dose interval over time. A total of 14 attributes were developed for the BWS. Thirteen attributes were selected from the themes that emerged from the qualitative interviews and one attribute was included after review with the research team. Attributes related to patient characteristics included JIA subtype, time in remission, history or presence of joint damage or erosive disease, how challenging it was to achieve remission, and history of flares. Contextual attributes included accessibility of biologics and willingness to taper biologics.
Conclusion
This study contributes to the limited literature on pediatric rheumatologists’ approaches to treatment decision making for biologics in JIA and identifies attributes that affect the decision to both initiate and taper. Further research is planned to implement the BWS survey to understand the importance of the attributes identified. Additional investigation is required to determine if these characteristics align with patient and parent preferences.
Similar content being viewed by others
References
Moorthy LN, Peterson MG, Hassett AL, Lehman TJ. Burden of childhood-onset arthritis. Pediatr. Rheumatol. 2010;8:20.
Vanoni F, Minoia F, Malattia C. Biologics in juvenile idiopathic arthritis: a narrative review. Eur J Pediatr. 2017;176:1147–53.
Ravelli A, Consolaro A, Horneff G, Laxer RM, Lovell DJ, Wulffraat NM, et al. Treating juvenile idiopathic arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2018;77:819–28.
Gartlehner G, Hansen RA, Jonas BL, Thieda P, Lohr KN. Biologics for the treatment of juvenile idiopathic arthritis: a systematic review and critical analysis of the evidence. Clin Rheumatol. 2008;27:67–76.
Prince FHM, van Suijlekom-Smit LWA. Cost of biologics in the treatment of juvenile idiopathic arthritis: a factor not to be overlooked. Pediatr Drugs. 2013;15:271–80.
Bernatsky S, Duffy C, Malleson P, Feldman DE, St Pierre Y, Clarke AE. Economic impact of juvenile idiopathic arthritis. Arthritis Rheum. 2007;57:44–8.
Kip MMA, de Roock S, Currie G, Marshall DA, Grazziotin LR, Twilt M, et al. Costs of medication use among patients with juvenile idiopathic arthritis in the Dutch healthcare system. Expert Rev Pharmacoecon Outcomes Res. 2021;21(5):975–84.
Halyabar O, Mehta J, Ringold S, Rumsey DG, Horton DB. Treatment withdrawal following remission in Juvenile Idiopathic Arthritis: a systematic review of the literature. Pediatr Drugs. 2019;21:469–92.
Horton DB, Onel KB, Beukelman T, Ringold S. Attitudes and approaches for withdrawing drugs for children with clinically inactive nonsystemic JIA: a survey of the childhood arthritis and rheumatology research alliance. J Rheumatol. 2017;44:352–60.
Broughton T, Armon K. Defining juvenile idiopathic arthritis remission and optimum time for disease-modifying anti-rheumatic drug withdrawal: why we need a consensus. Paediatr Drugs. 2012;14:7–12.
Lipstein EA, Brinkman WB, Sage J, Lannon CM, Morgan DE. Understanding treatment decision making in juvenile idiopathic arthritis: a qualitative assessment. Pediatr Rheumatol. 2013;11:34.
Guzman J, Gómez-Ramírez O, Jurencak R, Shiff NJ, Berard RA, Duffy CM, et al. What matters most for patients, parents, and clinicians in the course of juvenile idiopathic arthritis? A qualitative study. J Rheumatol. 2014;41:2260–9.
Lipstein EA, Dodds CM, Lovell DJ, Denson LA, Britto MT. Making decisions about chronic disease treatment: a comparison of parents and their adolescent children. Health Expect. 2016;19:716–26.
Horton DB, Salas J, Wec A, Kohlheim M, Kapadia P, Beukelman T, et al. Making decisions about stopping medicines for well-controlled juvenile idiopathic arthritis: a mixed-methods study of patients and caregivers. Arthritis Care Res. 2021;73:374–85.
Lipstein EA, Britto MT. Evolution of pediatric chronic disease treatment decisions: a qualitative, longitudinal view of parents’ decision-making process. Med Decis Making. 2015;35:703–13.
Kuijper TM, Folmer R, Stolk EA, Hazes JMW, Luime JJ. Doctors’ preferences in de-escalating DMARDs in rheumatoid arthritis: a discrete choice experiment. Arthritis Res Ther. 2017;19:78.
Burnett HF, Regier DA, Feldman BM, Miller FA, Ungar WJ. Parents’ preferences for drug treatments in juvenile idiopathic arthritis: a discrete choice experiment. Arthritis Care Res. 2012;64:1382–91.
Soekhai V, de Bekker-Grob EW, Ellis AR, Vass CM. Discrete choice experiments in health economics: past, present and future. Pharmacoeconomics. 2019;37:201–26.
Cheung KL, Wijnen BFM, Hollin IL, Janssen EM, Bridges JF, Evers SMAA, et al. Using best-worst scaling to investigate preferences in health care. Pharmacoeconomics. 2016;34:1195–209.
Flynn TN, Louviere JJ, Peters TJ, Coast J. Best–worst scaling: what it can do for health care research and how to do it. J Health Econ. 2007;26:171–89.
Bridges JFP, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, et al. Conjoint analysis applications in health—a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health J Int Soc Pharmacoecon Outcomes Res. 2011;14:403–13.
Hollin IL, Craig BM, Coast J, Beusterien K, Vass C, DiSantostefano R, et al. Reporting formative qualitative research to support the development of quantitative preference study protocols and corresponding survey instruments: guidelines for authors and reviewers. Patient. 2020;13:121–36.
Hazlewood GS, Loyola-Sanchez A, Bykerk V, Hull PM, Marshall D, Pham T, et al. Patient and rheumatologist perspectives on tapering DMARDs in rheumatoid arthritis: a qualitative study. Rheumatology (Oxford). 2022;61(2):606–16. https://doi.org/10.1093/rheumatology/keab330.
Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3:77–101.
Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–57.
Tymms K, Zochling J, Scott J, Bird P, Burnet S, de Jager J, et al. Barriers to optimal disease control for rheumatoid arthritis patients with moderate and high disease activity. Arthritis Care Res. 2014;66:190–6.
Vavricka SR, Radivojevic S, Manser CN, Frei P, Burri E, Fried M, et al. Addressing current treatment challenges in Crohn’s disease in real life: a physician’s survey. Dig Liver Dis. 2014;46:1066–71.
Anink J, Otten MH, Gorter SL, Prince FHM, van Rossum MAJ, van den Berg JM, et al. Treatment choices of paediatric rheumatologists for juvenile idiopathic arthritis: etanercept or adalimumab? Rheumatology. 2013;52:1674–9.
Kearsley-Fleet L, Davies R, Baildam E, Beresford MW, Foster HE, Southwood TR, et al. Factors associated with choice of biologic among children with Juvenile Idiopathic Arthritis: results from two UK paediatric biologic registers. Rheumatology. 2016;55:1556–65.
Shenoi S, Nanda K, Schulert GS, Bohnsack JF, Cooper AM, Edghill B, et al. Physician practices for withdrawal of medications in inactive systemic juvenile arthritis, Childhood Arthritis and Rheumatology Research Alliance (CARRA) survey. Pediatr Rheumatol. 2019;17:48.
Leblanc CMA, Lang B, Bencivenga A, Chetaille A-L, Dancey P, Dent P, et al. Access to biologic therapies in Canada for children with juvenile idiopathic arthritis. J Rheumatol J Rheumatol. 2012;39:1875–9.
Acknowledgements
This study is part of the Understanding Childhood Arthritis Network (UCAN) CURE consortia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the Canadian Institutes for Health Research (Canada) [Grant number 381280]; Genome Canada (Canada) [OGI-150]; ZonMw (The Netherlands); and ReumaNederland (The Netherlands). DAM is supported by the Arthur J.E. Child Chair in Rheumatology and a Canada Research Chair in Health Systems and Services Research (2008–2018). SB is supported by the Husky Energy Chair in Child and Maternal Health and the Alberta Children’s Hospital Foundation Chair in Pediatric Research. RSMY is supported by the Hak-Ming and Deborah Chiu Chair in Paediatric Translational Research, The Hospital for Sick Children, University of Toronto. GSH is supported by a Canadian Institutes of Health Research (CIHR) New Investigator Award.
Conflicts of interest/competing interests
Gillian R. Currie, Tram Pham, Marinka Twilt, Maarten J. IJzerman, Pauline M. Hull, Michelle M.A. Kip, Susanne M. Benseler, Glen S. Hazlewood, Rae S.M. Yeung, Nico M. Wulffraat, Joost F. Swart, Sebastian J. Vastert, and Deborah A. Marshall declare that they have no conflicts of interest relevant to the contents of this article.
Ethical approval
Ethics approval was obtained from the Conjoint Health Research Ethics Board at the University of Calgary (REB19-0360).
Availability of data and material
The ethics approval and consent for this study preclude the sharing of the raw data.
Code availability
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by GRC, TP, and PMH. The first draft of the manuscript was written by TP, GRC, and DAM. All authors commented on the manuscript, and all authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Currie, G.R., Pham, T., Twilt, M. et al. Perspectives of Pediatric Rheumatologists on Initiating and Tapering Biologics in Patients with Juvenile Idiopathic Arthritis: A Formative Qualitative Study. Patient 15, 599–609 (2022). https://doi.org/10.1007/s40271-022-00575-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40271-022-00575-x