Abstract
Recently, an increasing number of cases with delayed cutaneous reaction after immunization with mRNA-based vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been reported. This adverse reaction, which is considered a delayed-type or T cell-mediated hypersensitivity reaction, has been described for the Moderna (mRNA-1273) and Comirnaty (Pfizer/BioNTech, BNT162b2) vaccines. We describe a delayed large local cutaneous reaction in a patient who received the viral vector vaccine Vaxzevria (ChAdOx1-S, AstraZeneca). The time course and clinical symptoms of delayed skin reaction after mRNA vaccines have a similar pattern that we recognized in our patient after Vaxzevria vaccination. This phenomenon has not been described in the Vaxzevria clinical trials and is to our knowledge the first report of this adverse reaction to a vector-based SARS-CoV-2 vaccine. With this, we hope to raise awareness about delayed injection site reactions that also occur after viral vector vaccines and to encourage additional reporting and patient education regarding the cutaneous reactions after coronavirus disease 2019 (COVID-19) vaccination.
Similar content being viewed by others
mRNA vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been described to cause a delayed injection site reaction after vaccination (“COVID arm”). |
We describe a case of delayed cutaneous reaction after immunization with the viral vector vaccine Vaxzevria. |
Patient education is key to allaying concerns about side effects of COVID-19 vaccination and encouraging completion of vaccination schedules. |
1 Introduction
In January 2021, the European Medicines Agency (EMA) authorized use of the viral vector coronavirus disease 2019 (COVID-19) vaccine Vaxzevria (ChAdOx1-S, AstraZeneca) in the European Union. Given the scale-up of mass vaccination campaigns across the world, it is likely that new adverse reactions will occur that were not reported in the initial trials.
2 Case Presentation
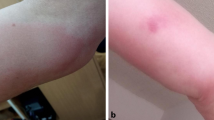
We have observed a delayed large local skin reaction to the first dose of Vaxzevria in a 62-year-old, white female. Except for cat allergy, no history of allergy was reported. Previous vaccinations had been well tolerated without remarkable skin or systemic reactions. Following the resolution of initial local and systemic symptoms after vaccination, the delayed reaction had an onset on day 10 that appeared near the injection site. The appearance of the reaction was an erythema with a maximum diameter of 10 cm on day 14 after vaccination (Fig. 1). Considerable induration and pain near the injection site was reported. Besides the local erythema, no further cutaneous symptoms in other localizations were observed. The symptoms cleared up after 15 days and fully resolved over the following 3 days. No systemic symptoms occurred with delayed local reaction. The patient did not require treatment for reaction. A biopsy was not performed.
Photographs of the delayed large local cutaneous reaction that first occurred on day 10 after vaccination with Vaxzevria. The cutaneous findings from A day 11 to E day 15 after vaccination are depicted. Morphologic characteristics included A an erythematous macula with initially indistinct margin and follicular accentuated. The macula does not blanch with pressure. B The macula is extending centripetally and horizontally, at first without central fading, then C becoming confluent (D–E) and with central fading in the course. Small skin wrinkles become visible again. The macula is surrounded by a pale peripheral margin. The symptoms resolved from day 15
The complete post-vaccination course was as follows: On day 0, the vaccination with Vaxzevria took place and no immediate reaction to the vaccine was observed. On day 1 after vaccination, the patient reported elevated temperature, malaise, and fatigue. The symptoms of systemic reaction resolved on day 2, but a slight soreness near the injection site was reported. On day 3, an erythema with a 5-cm diameter, warmth, and swelling was observed near the injection site. On days 4–6, local symptoms remitted and completely resolved on day 7–9 except for a palpable nodule in the deep soft tissue. On day 10, a new erythema recurred near the injection site and spread to a maximum diameter of 10 cm on days 11–14, accompanied by large, localized swelling and induration. From day 15, the erythema was regressive and had completely resolved by day 18. Photographs show the skin reaction over time, with varied size and severity of the local findings between day 10 and 15 (Fig. 1).
In the light of changing data on COVID-19 vaccines [1], the German Standing Committee on Vaccination (STIKO) has recently updated its recommendations regarding boost vaccination after primary immunization with Vaxzevria [2]. Therefore, the patient received a heterologous boost with Comirnaty 6 weeks after the Vaxzevria vaccination. The second vaccination was well tolerated. Apart from a slight soreness in the arm, no local or systemic reactions occurred.
3 Discussion
In the Vaxzevria clinical trials, a delayed local large reaction to the vaccine has not been described. In the phase 1/2 trial of Vaxzevria, local site reactions were only recorded for 7 days following vaccination [3]. It is therefore possible that the observation period was too short to report the delayed injection site reactions. Likewise, in the interim primary efficacy analysis of four phase 3 randomized controlled trials of ChAdOx1-S, no case with delayed cutaneous reaction was reported [4].
On the other hand, a similar phenomenon as in our patient has been described multiple times for the mRNA-based Moderna (mRNA-1273) vaccine and to a lesser extent for Comirnaty (Pfizer/BioNTech, BNT162b2) [5,6,7,8]. In the Moderna phase 3 trial, delayed injection site reaction occurred in 244 participants (0.8%) after the first dose of Moderna vaccine and in 68 participants (0.2%) after the second dose [5]. The delayed injection site reaction was defined as reaction on or after day 8.
The International COVID-19 Dermatology Registry started capturing skin reactions to COVID-19 vaccines in January 2021 and just recently published 414 cases with skin reactions to Comirnaty and Moderna vaccines [7]. They defined delayed large local reactions as occurrence after 4 or more days after vaccination. The most common cutaneous reaction to Moderna vaccination was delayed large local reaction (n = 175 first dose, n = 31 second dose). Interestingly, they also reported cases with local large reaction after administration of Comirnaty (n = 5 first dose, n = 7 second dose). Of note, no cases of anaphylaxis or other serious adverse events after subsequent vaccination following delayed major local reactions to Moderna or Comirnaty vaccines were described [7].
The time course and clinical symptoms of delayed major skin reaction after vaccination with Moderna or Comirnaty have a similar pattern that we recognized in our patient after Vaxzevria vaccination. To the best of our knowledge, this is the first description of a patient with delayed large local reaction to the vector-based Vaxzevria vaccine.
We suspect a delayed-type or T cell-mediated hypersensitivity reaction as it was proposed for the mRNA vaccines [6]. A skin-biopsy specimen obtained from a patient with delayed large local reaction after Moderna vaccination showed findings consistent with delayed-type or T cell-mediated hypersensitivity, supporting this hypothesis [6]. Although the aetiology of these reactions is unclear, delayed-type hypersensitivity reactions to excipient ingredients of vaccines have been reported previously [7].
While polysorbate 80 is the excipient in Vaxzevria and Johnson & Johnson (Janssen) vaccines, polyethylene glycol (PEG) 2000 is used in Moderna and Comirnaty vaccines. Polysorbate 80 is structurally similar to PEGs, and cross-reactivity of PEGs with polysorbate 80 has been described [9]. PEG 2000 is a substance not used before in vaccine development [10], and it is hypothesized that delayed localized cutaneous reactions to Moderna and Comirnaty vaccines may be associated with T cell responses to the new vaccine excipient [11]. For polysorbate 80, delayed cutaneous reactions have long been described after intramuscular injections [12] and may be the culprit antigen in this case.
Thousands of medications contain either PEGs or polysorbates [9]. Our patient is taking 5 mg olmesartan (ALIUD Pharma GmbH) once daily. This drug contains PEG 400, but neither polysorbate 80 nor PEG 2000. Therefore, amplification of hypersensitivity reaction due to concomitant drug intake is unlikely. However, cross-reactive hypersensitivity cannot be ruled out without allergy skin testing.
4 Conclusion
We report a patient with delayed large cutaneous hypersensitivity reaction after first vaccination with the vector-based Vaxzevria vaccine. Given that neither local injection site reactions nor delayed-type hypersensitivity reactions are contraindications to subsequent vaccination, our patient was encouraged to complete her vaccination course. Based on updated recommendations of German STIKO, the patient received a heterologous boost with Comirnaty. Allergy testing of patients with “COVID arm” using vaccine components may be informative. With this correspondence, we would like to raise awareness about delayed injection site reactions occurring also after viral vector vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and hope to encourage additional reporting and patient education regarding the cutaneous reactions after COVID-19 vaccination.
References
Shaw RH, Stuart A, Greenland M, Liu X, Nguyen Van-Tam JS, Snape MD. Heterologous prime-boost COVID-19 vaccination: initial reactogenicity data. Lancet. 2021;397(10289):2043–6.
German Standing Committee on Vaccination (STIKO). Mitteilung der STIKO zur COVID-19-Impfung: Impfabstand und heterologes Impfschema nach Erstimpfung mit Vaxzevria (1.7.2021). https://www.rki.de/DE/Content/Kommissionen/STIKO/Empfehlungen/PM_2021-07-01.html. Accessed 8 July 2021.
Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–78.
Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111.
Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–16.
Blumenthal KG, Freeman EE, Saff RR, Robinson LB, Wolfson AR, Foreman RK, et al. Delayed large local reactions to mRNA-1273 vaccine against SARS-CoV-2. N Engl J Med. 2021;384(13):1273–7.
McMahon DE, Amerson E, Rosenbach M, Lipoff JB, Moustafa D, Tyagi A, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85:46–55.
Ramos CL, Kelso JM. “COVID Arm”: very delayed large injection site reactions to mRNA COVID-19 vaccines. J Allergy Clin Immunol Pract. 2021;9:2480–1.
Stone CA Jr, Liu Y, Relling MV, Krantz MS, Pratt AL, Abreo A, et al. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J Allergy Clin Immunol Pract. 2019;7(5):1533-40.e8.
Kounis NG, Koniari I, de Gregorio C, Velissaris D, Petalas K, Brinia A, et al. Allergic reactions to current available COVID-19 vaccinations: pathophysiology, causality, and therapeutic considerations. Vaccines (Basel). 2021;9(3):221.
Johnston MS, Galan A, Watsky KL, Little AJ. Delayed localized hypersensitivity reactions to the Moderna COVID-19 vaccine: a case series. JAMA Dermatol. 2021;157(6):716–20.
Shelley WB, Talanin N, Shelley ED. Polysorbate 80 hypersensitivity. Lancet. 1995;345(8960):1312–3.
Acknowledgements
We gratefully acknowledge Prof. Manuel Cornely's support in describing the dermatologic findings of the delayed cutaneous reaction in our patient. We also thank Jan Thielebeule for technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors received no specific funding for this work.
Conflicts of interest/Competing interests
RS, SS, MP, and WP have nothing to disclose. OAC reports grants and personal fees from Actelion, personal fees from Allecra Therapeutics, personal fees from Al-Jazeera Pharmaceuticals, grants and personal fees from Amplyx, grants and personal fees from Astellas, grants and personal fees from Basilea, personal fees from Biosys, grants and personal fees from Cidara, grants and personal fees from Da Volterra, personal fees from Entasis, grants and personal fees from F2G, grants and personal fees from Gilead, personal fees from Grupo Biotoscana, personal fees from IQVIA, grants from Janssen, personal fees from Matinas, grants from Medicines Company, grants and personal fees from MedPace, grants from Melinta Therapeutics, personal fees from Menarini, grants and personal fees from Merck/MSD, personal fees from Mylan, personal fees from Nabriva, personal fees from Noxxon, personal fees from Octapharma, personal fees from Paratek, grants and personal fees from Pfizer, personal fees from PSI, personal fees from Roche Diagnostics, grants and personal fees from Scynexis, personal fees from Shionogi, grants from DFG, German Research Foundation, grants from German Federal Ministry of Research and Education, and grants from Immunic, outside the submitted work.
Ethics approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
The patient gave her permission to use clinical information and photographs in the publication.
Availability of Data and Material
All data generated or analysed during this study are included in this published article.
Code Availability
Not applicable.
Author contributions
RS drafted the initial version of the manuscript, prepared the figure, and reviewed and approved the final version of the manuscript. SS contributed to manuscript preparation and reviewed and approved the final version of the manuscript. MP provided the case description and photographs and reviewed and approved the final version of the manuscript. WP provided the case description and photographs and reviewed and approved the final version of the manuscript. OAC conceived the study idea and reviewed and approved the final version of the manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Sprute, R., Schumacher, S., Pauls, M. et al. Delayed Cutaneous Hypersensitivity Reaction to Vaxzevria (ChAdOx1-S) Vaccine against SARS-CoV-2. Drugs R D 21, 371–374 (2021). https://doi.org/10.1007/s40268-021-00358-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40268-021-00358-z