Abstract
Background
It is unclear whether generics are as safe as brand-name drugs in cardiology. For public health surveillance purposes, we evaluated if switching from the brand-name losartan, valsartan, or candesartan impacted the occurrence of the following outcomes: emergency room (ER) consultations, hospitalizations, or death.
Study Design
This was a retrospective cohort study.
Methods
This study was conducted in the Quebec Integrated Chronic Disease Surveillance System, including healthcare administrative data of the population of Quebec, Canada. We included brand-name users of losartan, valsartan, or candesartan aged ≥ 66 years who had undergone ≥ 30 days of stable treatment on the brand-name drug prior to cohort entry (substitution time-distribution matching was used to prevent immortal time bias). Outcomes up to 1 year were compared between groups using multivariable Cox proportional hazards regression models (validity assumptions were verified).
Results
In our cohorts (losartan, n =15,783; valsartan, n =16,907; candesartan, n =26,178), mean age was 76–78 years, 59–66% were female, 90–92% had hypertension, and 13–15% had heart failure. Validity assumptions were violated for losartan only. For patients switched to generic valsartan, the hazard ratio (95% confidence interval) was 1.07 (0.99–1.14) for ER consultation, 1.26 (1.14–1.39) for hospitalization, and 1.01 (0.61–1.67) for death. The corresponding rates for candesartan were 1.00 (0.95–1.05), 0.96 (0.89–1.03), and 0.57 (0.37–0.88), respectively.
Conclusions
We observed an increased risk of hospitalizations for patients switched to generic valsartan, and a decreased risk of death for patients switched to generic candesartan, compared with those who continued taking the brand-name drug. The differences between generic and brand-name drugs may lead to some differences in public health outcomes, but this safety signal must be further studied using other cohorts and settings.
Similar content being viewed by others
Some differences in hospitalizations and mortality rates were found after generic substitution. |
This safety signal must be further studied using other cohorts and settings. |
1 Introduction
Substitution from brand-name to generic drugs is known to decrease pharmaceutical expenditures [1]. Direct cost savings from generic substitution are beneficial for both health care systems and payers. Apart from identical strength of the active ingredient, galenic form, route of administration, and indication, the bioavailability of generic versus brand-name drugs is similar. Briefly, in many countries such as Canada, a maximal 20% relative difference is allowed between a given oral generic drug and its corresponding brand-name (such as angiotensin II receptor blockers [ARBs]) to be judged bioequivalent by health authorities. The manufacturer does not need to demonstrate that its generic is bioequivalent to other generics on the market, nor does it need to demonstrate therapeutic equivalence with the corresponding brand-name; therapeutic equivalence is simply extrapolated from the brand-name clinical trial results [2,3,4].
The clinical impact of switching from brand-name to generics in ‘real-life’ conditions remains unknown. Indeed, several studies have been reporting an association between hospital visits and generic drug use [5,6,7,8,9]. Furthermore, the recent contaminated generic ‘sartans’ scandal affected millions of patients worldwide [10,11,12]. The equivalence and safety of brand-name to generic substitution may constitute a public health issue.
In this retrospective cohort study, we aimed to characterize the impact of generic ARB substitution on the occurrence of the following outcomes: emergency room [ER] consultations, hospitalizations, or all-cause mortality in an elderly population. As generic and brand-name drugs are considered bioequivalent, we hypothesized that we should not find any difference in time-to-event between patients who continued taking the brand-name drug versus those exposed to generic substitution.
2 Methods
2.1 Data Source
Data were extracted from the Quebec Integrated Chronic Disease Surveillance System (QICDSS) [13], including five medico-administrative files held by the public health insurance board in the province of Quebec (Canada) and the health ministry. Four of these files were used: (1) the health insurance registry; (2) hospitalizations; (3) medical services; and (4) pharmaceutical services. The latter included about 90% of citizens ≥ 65 years of age [13]. Data were linked by a unique anonymized identification number. This study was part of the continuous chronic disease surveillance mandate granted to the National Public Health Institute of Quebec (Institut national de santé publique du Québec; INSPQ) by the provincial Minister of Health and Social Services [14]. All surveillance activities of this mandate are approved by the provincial Ethics Committee of Public Health. No informed consent was required.
2.2 Study Design and Inclusion Criteria
This was a population-based, observational, retrospective cohort study [15]. Three cohorts were constituted of brand-name losartan, valsartan, or candesartan users once a respective brand-name’s patent expired and their respective generics were first reimbursed in the province of Quebec [6]. As each brand-name ARB went generic at different time points [6], the respective exposure period of each cohort differs. Inclusion criteria were (1) age ≥ 66 years; (2) ≥ 30-day use of the studied brand-name ARB prior to cohort entry; and (3) ≥ 15 days on the same dose of the brand-name drug immediately before the cohort entry (justification in the Electronic Supplementary Material [ESM]).
2.3 Drug Selection
All dosages of the three ARBs that were indicated to treat hypertension [16] and heart failure [17] and that lost their patent in 2010–2011 (losartan, valsartan, or candesartan) were selected and identified by their respective drug identification number (DIN) [6]. From 15 March 2012, there were eight generics of losartan on the market, but only three for valsartan (20 April 2011) and candesartan (6 July 2011) [6]. Pseudogenerics (also named ‘ultra-generics’) were excluded as they were waived from the comparative bioavailability studies (n =2 manufacturers of valsartan) [18]. In the pharmaceutical database, the start date and duration of each brand-name or generic drug claimed allowed for individual exposure and switch date to be precisely obtained. The exact generic drug start date for exposure group classification was corrected by a validated methodology (continuous multiple interval measure of oversupply) [6, 19].
2.4 Exposure to Generic Substitution
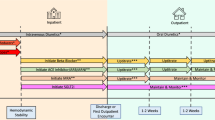
Exposure was defined by switching from the brand-name drug to a corresponding generic version within 1 year after the first generic version had been marketed (Fig. 1). Patients who continued taking the brand-name drug within 1 year of reimbursement of the generics were considered unexposed.
2.5 Cohort Entry (Index Date)
Switching from a brand-name to a generic drug (namely ‘generic substitution’) determined the date of cohort entry for exposed patients (Fig. 1). Patients who did not switch (unexposed, continued taking the brand-name drug), cohort entry was attributed by time-distribution matching (named ‘substitution time-distribution matching’ in this study; see the ESM) [20].
2.6 Outcomes
In separate models, we analyzed the times from cohort entry to predefined outcomes up to 1 year of follow-up: (1) ER consultation; (2) hospitalization; or (3) death of all-cause, in line with signal detection principles in pharmacovigilance and the public health surveillance mandate of the INSPQ.
2.7 Censoring Criteria
As we needed to associate a possible outcome with an active exposure, we censored the follow-up for (1) non-persistence to the study drug (a maximal grace period ≤ 7 days was allowed between two claims); (2) switching back to the brand-name drug (due to possible unmeasurable confounders in such a context); or (3) end of follow-up. For non-fatal outcomes, we additionally censored at death.
2.8 Statistical Analyses
Descriptive analyses were performed on the available covariables traditionally studied in public health surveillance and known as potential confounders in pharmacoepidemiology (age at cohort entry, sex, comorbidities within 1 year of cohort entry, concomitant drugs within 1 year of cohort entry, a geographical proxy of socioeconomic status [the material and social deprivation index at cohort entry] [21], duration of the brand-name drug utilization before generic substitution [cohort entry], ER consultations or hospitalizations within 60-days prior to cohort entry, region of residence, and the prescriber’s speciality at cohort entry), as well as outcomes for each cohort, to obtain proportions, means, standard deviations, or median and interquartile (IQR) ranges, where appropriate. Chi-squared tests, independent t tests, or Wilcoxon rank-sum tests were performed to assess the univariate statistical significance of differences between groups [22].
Multivariable models were adjusted for potential confounders, as previously listed. An exploratory 60-day endpoint compared the risk of outcomes (defined as a binary outcome: yes/no) up to 60 days after generic substitution by multivariable logistic regression models (providing adjusted odds ratio [OR] and 95% confidence interval [CI]) [22]. Multivariable Cox proportional hazards regression models (providing adjusted hazard ratios [HR] and 95% CIs) were used to compare time-to-event up to 1 year for those exposed versus unexposed to generic substitution. For apparent validity, both types of models were adjusted for all covariables. Validity of assumptions were verified (see ESM). Many other sensitivity analyses were conducted and all are described in the ESM. The significance level was set at p < 0.05. All analyses were performed using SAS Enterprise Guide 7.1 (SAS Institute Inc., Cary, NC, USA).
3 Results
A flowchart of the study patient selection process is presented in Fig. 2. A total of 58,868 patients were included in the study. In the cohort of losartan users, 14,673 patients were exposed to generic substitution and 1110 patients continued taking the brand-name drug (with the cohort entry date matched by substitution time-distribution). For valsartan, 3353 patients were exposed to generic substitution and 13,554 continued taking the brand-name drug, and for candesartan, 13,648 patients were exposed to generic substitution and 12,530 patients continued taking the brand-name drug. Pharmacokinetics features (bioavailability) and proportions of each ARB used by the study population are shown in Electronic Supplementary Table 1.
3.1 Patient Characteristics
Baseline characteristics of the losartan, valsartan, and candesartan cohorts are presented in Table 1. Depending on the cohort, mean age at cohort entry was 76–78 years, 59–66% were female, 90–92% had hypertension, and 13–15% had heart failure. Patients had three comorbidities and were using 10–11 concomitant drugs on average. Age, sex, prescriber’s speciality, occurrence of outcomes ≤ 60 days prior to cohort entry, number of comorbidities, number of concomitant drugs, socioeconomic status, region of residence, and previous experience with the brand-name drug prior to cohort entry varied between those exposed and unexposed to generic substitution, even though some differences could be interpreted as minimal.
3.2 Follow-Up Times and Validity of Cox Proportional Hazards Models
Due to the high likelihood of informative censoring and the violation of Cox proportional hazards model assumptions in the losartan cohort, no more analysis or interpretation of outcomes between groups was therefore considered for this drug (more details reported in the ESM).
3.3 Emergency Room Consultations
The crude proportion of ER consultations up to 1 year was higher for those exposed to generic valsartan and candesartan substitution (p <0.05 for valsartan only) (Table 2). After adjustment for potential confounders, the time to ER consultation up to 1 year was similar between groups for both drugs (Fig. 3). Furthermore, no difference was found between groups at the 60-day endpoint (valsartan, adjusted OR 1.08 [0.94–1.23]; candesartan 1.06 [0.97–1.16]) (Electronic Supplementary Fig. 1).
3.4 Hospitalizations
The crude proportion of hospitalizations up to 1 year was higher for those exposed to generic valsartan substitution only (Table 2). After adjustment for potential confounders, the time to hospitalization up to 1 year was shorter for those exposed to generic valsartan (HR 1.26 [1.14–1.39]), and similar for generic candesartan (Fig. 3). No difference in the risk of hospitalization was found between groups at the 60-day endpoint (valsartan, adjusted OR 1.16 [0.93–1.44]; candesartan, 1.02 [0.88–1.18]) (Electronic Supplementary Fig. 1).
3.5 Mortality
The crude proportion of death up to 1 year was 0.1% lower for those exposed to generic candesartan and valsartan substitution (p <0.05 for candesartan only) (Table 2). After adjustment for potential confounders, the time to death up to 1 year remained similar between groups for valsartan (Fig. 3). For candesartan, survival was better for those exposed versus unexposed to generic substitution (HR 0.57 [0.37–0.88]). No differences in the risk of death were found between groups at the 60-day endpoint (valsartan, adjusted OR 0.86 [0.34–2.20]; candesartan, 0.73 [0.35–1.50]) (Electronic Supplementary Fig. 1).
3.6 Sensitivity Analyses: Cohort Construction and Follow-Up
First, HRs at 1 year were reanalysed in each cohort, allowing ARB users to be ‘non-persistent’ (> 7 days between two claims) during the follow-up period, yielding similar results (data not shown). Second, we attributed a brand-name well-defined point in time for cohort entry rather than using substitution time-distribution matching. The results systematically conferred a clear advantage to patients switched to generics, who had fewer hospitalizations or ER consultations (a well-known immortal time bias [20], data not shown). Third, the impact of the 30-day use period criteria prior to cohort entry was compared with a longer period (60 days), which yielded similar results (data not shown). Finally, restricting the cohorts to only new persistent users (who used the studied brand-name for ≤ 60 days prior to cohort entry) revealed similar results (data not shown), reassuring that depletion of susceptible users would not confound the comparisons between groups.
4 Discussion
4.1 Major Findings
In this retrospective cohort study including 58,868 ARB users, we observed some differences in time-to-event between patients exposed to generic substitution versus those who continued taking the brand-name drug. Results suggest a higher risk of hospitalization following the switch to generic valsartan, but a lower risk of death following the switch to generic candesartan. To our knowledge, this is the first populational study assessing the safety of generic substitution per se by using a validated time-distribution matching method.
4.2 Possible Explanations for Different Outcomes
Higher unadjusted proportions of ER consultations and hospitalization for those switched to generic ARBs are quite coherent with what has been previously found in a time-series analysis of the same drugs (n =136,177) [6], as well as other time-series analyses of warfarin (n =280,158) [9] and clopidogrel (n =89,525) [5]. Once adjusted, and time-to-event taken into account, differences in outcomes persisted only for patients switched to generic valsartan (higher risk of hospitalizations following substitution). Even though they remain to be further studied and understood, these differences in outcomes could be explained by differences in bioavailability between generics and the brand-name valsartan (Electronic Supplementary Table 1) [23, 24]. Switching to a generic drug that is more or less bioavailable could potentially induce acute or delayed adverse drug reactions (i.e. dizziness, diarrhea, headache, coughing, hypotension, syncope, renal insufficiency, and hypokalemia if the generic version is more bioavailable than the brand-name drug) [2,3,4] and require physician assessment, while switching to a generic that is less bioavailable could lead to a lack of efficacy (i.e. hypertension, congestive heart failure, depending on the severity of the case), uncontrolled disease, and also require physician assessment. Unfortunately, it was not possible to accurately assess such specific outcomes with validated coding and sufficient statistical power using our dataset and study design. Unlike many versions of generic valsartan commercialized worldwide since 2012 [10,11,12] generics of valsartan used by our cohort have never been reported as contaminated by N-nitrosodimethylamine or N-nitrosodiethylamine, known as probable human carcinogens.
Of note, even though there is only a 0.1% difference in the proportion of mortality between patients switched to generic candesartan versus those who continued taking the brand-name drug, the relative risk of mortality (considering time to event) was lower following the switch to generic candesartan (HR 0.57 [0.37–0.88]). This could theoretically be plausible as (1) all generics of candesartan were more bioavailable compared with the brand-name drug (electronic Supplementary Table 1); and (2) candesartan is known to reduce the risk of death [23, 24]. With this hypothesis, patients may have benefit from switching to a more bioavailable generic candesartan (for survival). However, those results must be validated using a different study design, as residual immortal time bias, as well as residual confounders, cannot be dismissed totally [25]. Finally, mortality was 0.3% lower for patients exposed to generic losartan compared with the brand-name drug, but, as previously mentioned, this difference may not be valid due to the presence of an informative censoring bias for this drug only. Unfortunately, it is not possible to interpret the differences in outcomes for losartan users.
4.3 Other Possible Explanations
Apart from slight pharmacodynamic differences between generic and brand-name ARBs, our results may be hypothetically explained by (1) fewer generic pill polymorphisms (more recently manufactured generics being superior, i.e. less moisture in the pill, better disintegration time, etc., versus formerly manufactured brand-name drugs); [26] and (2) generics’ active ingredient polymorphisms showing better or lower affinity to angiotensin II receptors (which was not assessed prior to being licensed and has already been found to be different) [10, 11, 27]. Those patients who switched may have had mild symptoms that were managed out of hospital (therefore not captured in our data), resulting in drug adjustments (Table 2) and, maybe, lower mortality. Those differences (bioavailability, polymorphisms) could explain, at least in part, discrepancies in the results between valsartan and candesartan. There are a lot of unknowns, leading to more questions, and options for more academic research.
Our results contrast with two large systematic reviews and meta-analyses, concluding there are similarities in outcomes between brand-name and generic drugs in cardiology [28, 29]. However, even though well-conducted, these meta-analyses may have underestimated the true effect by including comparative bioavailability studies (≥ 50%), not designed or powered to detect a difference in efficacy or safety [18]. Other cohort studies that found similar rates of death in the population exposed to generic versus brand-name statins [30], or clopidogrel [31], used head-to-head comparison groups without assessing the effect of the substitution per se. The latter study avoided this period when the study population switched to generics very quickly, which is very tricky to study considering the dynamics of the switches once generics became available [6]. A study design such as ours, accounting for substitution time-distribution between those exposed and unexposed to generics, was never used in those studies.
Half the population using losartan converted to generics within 2 months of availability for publicly insured patients in the province of Quebec, but it took 1 year for valsartan and candesartan [6]. This explains the highest proportion of those exposed to generic losartan in our study. Higher rates of generic losartan switching could explain the discrepancy between follow-up times and possible informative censoring for this drug only. For valsartan and candesartan, the slower rate of switching to generics may explain the statistical stability and validity of Cox proportional hazards models, therefore reassuring the validity of the hazard ratios. Furthermore, among all three drugs, losartan is the only drug that had confirmed drug safety reactions associated with generic substitution, in the MedEffect Database (Health Canada surveillance system) [32]. Finally, the disproportionality of patients switched to generics of valsartan (Fig. 2) is explained by our method. As previously justified, patients who switched to pseudogenerics (n =15,758) were excluded as those generics were identical to the brand-name drug [18].
4.4 Strengths and Limitations
Even in cases of unbalanced exposure groups, our retrospective cohort study included patients (1) with similar survival patterns at baseline, and (2) who reached a proxy of brand-name steady-state prior to cohort entry, to assess the bioequivalence hypothesis as accurately as possible. Furthermore, the follow-up of each patient was censored if the drug was not renewed (claimed), to maximize our ability to associate the exposure with the outcomes of interest. Our study design respected the natural temporal sequence between exposure and outcome. In addition, unlike others [33], we were able to include all the Quebec elderly population, publicly covered and using selected ARBs and their respective generics, maximizing external validity and statistical power, to detect acute or delayed adverse events [34]. The main limitation of this public health surveillance study is the use of nonspecific outcomes. It is sufficient to detect a potential drug safety signal but insufficient to assess causality [35]. Among other limitations, the substitution time-distribution matching method may not have fully addressed the possible immortal time bias [25]. Considering the complexity and rapidity of the switch in the population, a time-series analysis may be as appropriate as such a retrospective cohort study [5, 6, 9]. Moreover, defining exposure as a binary time-varying covariate is often recommended for survival analyses [20]. Along with intent-to-treat and non-inferiority analysis, this method was fully thought through and finally dismissed as it would not have provided an answer to the study question (aiming to assess outcomes following generic substitution per se and not outcomes while taking the brand-name drug versus the generic). Another limitation relates to drug claims that do not ascertain drug ingestion; [36] the impact of concomitant pharmacological changes or access to a primary care network were not considered but could be associated with outcomes [37]. Even though hospitalizations, medical services, and pharmaceutical claims databases are considered highly reliable [36, 38, 39], errors are possible and may have led to some misclassification. Death rate is low, reducing the possibility of competitive risk of death, but perhaps also affecting the ability to draw firm conclusions on relative differences between groups. Bias by indication (whether or not patients would be switched to generics by the community pharmacist) cannot be fully excluded but most confounders are controlled in regression models. Several potential confounding variables were not available in our QICDSS (e.g. cardiovascular risk factors, physicians’ and patients’ beliefs, etc.), which could modulate data analysis and interpretation in different settings. Finally, our study only assessed the impact of switching from the brand-name drug to the bioequivalent generic. The impact of switching between generics may be greater as they are not required to be proven bioequivalent between each other (Electronic Supplementary Table 1) [2,3,4].
4.5 Implications
Despite all its challenges, a population-wide switch to generics is an interesting and important field to study. Clinical uncertainty regarding the safety of a generic substitution in cardiology remains. There is still work to be done with pharmaceutical policies to delineate the independent impact of switching to a bioequivalent, but not identical, generic drug, especially if it is suspected to affect mortality rates.
5 Conclusions
In this retrospective cohort study, we found an increased risk of hospitalizations for patients switched to generic valsartan and a decreased risk of death for patients switched to generic candesartan, compared with those who continued taking the brand-name drug. Is brand-name to generic substitution safe? Uncertainty remains due to insufficient evidence. This signal should therefore be considered as exploratory. It merits in-depth clinical investigation as unmeasured/unavailable factors could partly explain these findings. Nevertheless, differences between generic and brand-name drugs may lead to some differences in public health outcomes, and this safety signal must be further studied using other cohorts and settings.
References
Gouvernement du Québec. Mesure d’économie concernant les médicaments - Nouvelles règles concernant le recours à la mention ne pas substituer. 2015. https://www.ramq.gouv.qc.ca/SiteCollectionDocuments/professionnels/infolettres/2015/info265-4.pdf. Accessed 20 Apr 2020.
Apotex inc. Monographie de produit APO-LOSARTAN. 2013. https://www.canada.ca/fr/sante-canada/services/medicaments-produits-sante/medicaments/base-donnees-produits-pharmaceutiques.html. Accessed 18 Nov 2015.
Mylan Pharmaceuticals ULC. Monographie de produit Mylan-valsartan. 2015. https://www.canada.ca/fr/sante-canada/services/medicaments-produits-sante/medicaments/base-donnees-produits-pharmaceutiques.html. Accessed 18 Nov 2015.
Sandoz Canada Inc. Monographie de produit Sandoz Candésartan (candésartan). 2016. https://www.canada.ca/fr/sante-canada/services/medicaments-produits-sante/medicaments/base-donnees-produits-pharmaceutiques.html. Accessed 30 Sept 2017.
Leclerc J, Blais C, Rochette L, Hamel D, Guenette L, Poirier P. Did generic clopidogrel commercialization affect trends of er consultations and hospitalizations in the population treated with clopidogrel? Drugs Aging. 2019;36(8):759–68.
Leclerc J, Blais C, Rochette L, Hamel D, Guénette L, Poirier P. Impact of the commercialization of three generic angiotensin II receptor blockers on adverse events in Quebec, Canada: a population-based time series analysis. Circ Cardiovasc Qual Outcomes. 2017;10:1–9.
Ghate SR, Biskupiak JE, Ye X, Hagan M, Kwong WJ, Fox ES, Brixner DI. Hemorrhagic and thrombotic events associated with generic substitution of warfarin in patients with atrial fibrillation: a retrospective analysis. Ann Pharmacother. 2011;45:701–12.
Kwong WJ, Kamat S, Fang C. Resource use and cost implications of switching among warfarin formulations in atrial fibrillation patients. Ann Pharmacother. 2012;46:1609–16.
Leclerc J, Blais C, Rochette L, Hamel D, Guénette L, Poirier P. Trends in hospital visits for generic and brand-name warfarin users in Quebec, Canada; a population-based time series analysis. Am J Cardiovasc Drugs. 2018;19(3):287–97.
Leclerc J. Recall of NDMA-contaminated pseudogeneric valsartan; best generics finally no better than others? Can J Cardiol. 2018;34(1370):e13.
Extance A. Sartan drug contamination brings cancer uncertainty. 2018. https://www.chemistryworld.com/news/sartan-drug-contamination-brings-cancer-uncertainty/3009849.article. Accessed 3 Apr 2019.
European Medicine Agency. Update on review of recalled valsartan medicines. 2018. http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2018/08/news_detail_003000.jsp&mid=WC0b01ac058004d5c1. Accessed 6 Aug 2018.
Blais C, Jean S, Sirois C, Rochette L, Plante C, Larocque I, et al. Quebec Integrated Chronic Disease Surveillance System (QICDSS), an innovative approach. Chronic diseases and injuries in Canada. 2014;34:226–35.
Saint-Laurent D, Blais C, Jean S, Sirois C, Rochette L and Émond V. Le modèle québécois de surveillance des maladies chroniques basé sur l’utilisation des données médico-administratives jumelées. Bulletin épidémiologique hebdomadaire. 2013;Hors-série:4-8.
Strom BL. Chapter 2. Study designs available for pharmacoepidemiologic studies. In: Strom BL, Kimmel SE, Hennessy S, editors. Textbook of pharmacoepidemiology. 2nd ed. Chichester: Wiley Blackwell; 2013. p. 456.
Programme d’éducation canadien sur l’hypertension. Guide de pratique clinique d’Hypertension Canada sur la prise en charge de l’hypertension. 2017. http://guidelines.hypertension.ca/ressources-francaises/. Accessed 11 Sept 2017.
Ezekowitz JA, O’Meara E, McDonald MA, Abrams H, Chan M, Ducharme A, et al. 2017 Comprehensive Update of the Canadian Cardiovascular Society Guidelines for the Management of Heart Failure. Can J Cardiol. 2017;33(11):1342–433.
Santé Canada. Ligne directrice - Normes en matière d’études de biodisponibilités comparatives: Formes pharmaceutiques de médicaments à effets systémiques. Médicaments et produits de santé. 2018. https://www.canada.ca/fr/sante-canada/services/medicaments-produits-sante/medicaments/demandes-presentations/lignes-directrices/biodisponibilite-bioequivalence/normes-matiere-etudes-biodisponibilite-comparatives-formes-pharmaceutiques-medicaments-effets-systemiques.html. Accessed 29 Oct 2018.
Morningstar BA, Sketris IS, Kephart G, Sclar DA. Variation in pharmacy prescription refill adherence measures by type of oral antihyperglycaemic drug therapy in seniors in Nova Scotia, Canada. J Clin Pharm Ther. 2002;27:213–20.
Zhou Z, Rahme E, Abrahamowicz M, Pilote L. Survival bias associated with time-to-treatment initiation in drug effectiveness evaluation: a comparison of methods. Am J Epidemiol. 2005;162:1016–23.
Pampalon R, Hamel D, Gamache P. A comparison of individual and area-based socio-economic data for monitoring social inequalities in health. Health Rep. 2009;20:85–94.
Vittinghoff E. Regression methods in biostatistics: linear, logistic, survival, and repeated measures models. New York: Springer; 2012.
Novartis Pharmaceuticals Canada Inc. Monographie de produit DIOVAN (valsartan). 2015. https://www.canada.ca/fr/sante-canada/services/medicaments-produits-sante/medicaments/base-donnees-produits-pharmaceutiques.html. Accessed 18 Nov 2015.
AstraZeneca Inc. Monographie ATACAND (candésartan cilexétil). 2016. https://www.canada.ca/fr/sante-canada/services/medicaments-produits-sante/medicaments/base-donnees-produits-pharmaceutiques.html. Accessed 30 Sept 2017.
Karim ME, Gustafson P, Petkau J, Tremlett H. Comparison of Statistical Approaches for Dealing With Immortal Time Bias in Drug Effectiveness Studies. Am J Epidemiol. 2016;184:325–35.
Singhal D, Curatolo W. Drug polymorphism and dosage form design: a practical perspective. Adv Drug Deliv Rev. 2004;56:335–47.
Meredith PA. Potential concerns about generic substitution: bioequivalence versus therapeutic equivalence of different amlodipine salt forms. Curr Med Res Opin. 2009;25:2179–89.
Manzoli L, Flacco ME, Boccia S, D’Andrea E, Panic N, Marzuillo C, et al. Generic versus brand-name drugs used in cardiovascular diseases. Eur J Epidemiol. 2016;31:351–68.
Kesselheim A, Misono AS, Lee JL, Stedman MR, Brookhart MA, Choudhry NK, et al. Clinical equivalence of generic and brand-name drugs used in cardiovascular disease: a systematic review and meta-analysis. JAMA. 2008;300:2514–26.
Jackevicius C, Tu JV, Krumholz HM, Austin PC, Ross JS, Stukel TA, et al. Comparative effectiveness of generic atorvastatin and lipitor(r) in patients hospitalized with an acute coronary syndrome. J Am Heart Assoc. 2016;5(4):e003350.
Ko DT, Krumholz HM, Tu JV, Austin PC, Stukel TA, Koh M, et al. Clinical outcomes of plavix and generic clopidogrel for patients hospitalized with an acute coronary syndrome. Circ Cardiovasc Qual Outcomes. 2018;11:e004194.
Gouvernement du Canada. Adverse reaction and medical device problem reporting. 2017. https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada/adverse-reaction-reporting.html. Accessed 26 Sept 2017.
Alatawi Y, Rahman M, Cheng N, Qian J, Hansen R, Seoane-Vazquez E, et al. Brand vs. generic adverse event reporting patterns: an authorized generic-controlled evaluation of cardiovascular medications. J Am Pharm Assoc. 2017;57.
Pan GJD, Lindquist M, Gelperin K. Chapter 7. Postmarketing spontaneous pharmacovigilance reporting systems. In: Strom BL, Kimmel SE, Hennessy S, editors. Textbook of pharmacoepidemiology. 2nd ed. Chichester: Wiley; 2013. p. 456.
Strom BL, Kimmel SE, Hennessy S. Textbook of pharmacoepidemiology. Chichester: Wiley Blackwell; 2013.
Tamblyn R, Lavoie G, Petrella L, Monette J. The use of prescription claims databases in pharmacoepidemiological research: the accuracy and comprehensiveness of the prescription claims database in Quebec. J Clin Epidemiol. 1995;48:999–1009.
McAlister FA, Bakal JA, Green L, Bahler B, Lewanczuk R. The effect of provider affiliation with a primary care network on emergency department visits and hospital admissions. Can Med Assoc J. 2018;190:E276–84.
Lambert L, Blais C, Hamel D, Brown K, Rinfret S, Cartier R, et al. Evaluation of care and surveillance of cardiovascular disease: can we trust medico-administrative hospital data? Can J Cardiol. 2012;28:162–8.
Wilchesky M, Tamblyn RM, Huang A. Validation of diagnostic codes within medical services claims. J Clin Epidemiol. 2004;57:131–41.
Acknowledgements
This study was conducted at the National Public Health Institute of Quebec (INSPQ) as part of the continuous chronic disease surveillance mandate granted by the provincial Minister of Health and Social Services. Dr Leclerc received a PhD studentship from the Institut universitaire de cardiologie et de pneumologie de Québec during this study. Dr Poirier is a senior clinical researcher of the Fonds de recherche du Québec-Santé (FRQ-S). Dr Guénette holds a Junior-1 clinical researcher salary award from the FRQ-S in partnership with the Société québécoise d’hypertension artérielle (SQHA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr Poirier has received honorarium for continuing medical education/consulting/expert events from Abbott Vascular, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Janssen, Merck, Novartis, NovoNordisk, Pfizer, Roche, Sanofi-Aventis, Servier, and Valeant. The industry has no regard/power in the decisions made through this project. Dr Leclerc is a Professor of Nursing at Université du Québec à Trois-Rivières. Within her role of Professor, she provides continuous medical education sessions for health care professionals, accredited by the Fédération des médecins omnipraticiens du Québec and its local affiliates, and statistical expertise on Data Safety Monitoring Board Committees managed by JSS Medical Research, a contract research organization that executes clinical trials/studies for pharmaceutical and biotechnology companies, as well as universities and hospitals. Claudia Blais, Louis Rochette, Denis Hamel, Line Guénette, and Claudia Beaudoin have no potential conflict of interest.
Ethics and Funding
No funding was received for this study as the project is part of the continuous chronic disease surveillance mandate in Quebec, Canada. All surveillance activities of this mandate are approved by the provincial Ethic Committee of Public Health. No informed consent was required.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Leclerc, J., Blais, C., Rochette, L. et al. Public Health Outcomes May Differ After Switching from Brand-Name to Generic Angiotensin II Receptor Blockers. Drugs R D 20, 135–145 (2020). https://doi.org/10.1007/s40268-020-00307-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40268-020-00307-2






