Abstract
Objective
The aim was to assess patterns in reporting of adverse drug reactions (ADRs) via the Yellow Card (YC) Scheme following a Scottish community pharmacy patient YC promotional campaign (January–February 2011).
Methods
YC data were obtained from the Medicines and Healthcare Products Regulatory Agency (MHRA) [January 2009–February 2012]. The impact of the campaign on YC reporting rates was assessed by comparing YC submission rates before and after the intervention, using the segmented regression of interrupted time-series analysis.
Results
The mean weekly reported ADRs [excluding general practitioner (GP) reports] before, during, and after the campaign were 0.029, 0.019, and 0.023 (per 10,000 inhabitants), respectively. In relation to patients’ YC reporting, the mean weekly patient-reported ADRs before, during, and after the campaign in Scotland were 0.005, 0.002, and 0.004 (per 10,000 inhabitants), respectively. The time-series analysis for monthly reported ADRs in Scotland (excluding GP reports) demonstrated no statistically significant level change (p = 0.706) and no significant trend change (p = 0.509) post-campaign. Similarly, there was no statistically significant level change (p = 0.983) and no significant trend change (p = 0.591) in patient YC reporting.
Conclusions
The campaign had no statistically significant impact on influencing the reporting of ADRs. This study adds to a growing body of required information in this area, and suggests improvements if future patient ADR-reporting promotional campaigns are to be considered; the cost-effectiveness of such efforts requires further research. It is recommended that any similar future campaigns should include qualitative attitudinal data collection and evaluation to help further explore this more robustly.
Similar content being viewed by others
Introduction
In the UK, the Medicines and Healthcare products Regulatory Agency (MHRA) runs the Yellow Card (YC) Scheme as one of the essential components of its pharmacovigilance strategy to monitor the safety of medicines over their lifespan and to detect any unknown potential adverse drug reactions (ADRs) from signals generated from the individual case reports submitted by healthcare professionals (HCPs) or the public (i.e. patients, carers or parents). This system has been in place since 1964, and the spontaneous individual case reports underpinning this system form the cornerstone of pharmacovigilance around the world.
ADRs are considered a major public health problem in clinical practice worldwide and have been associated with increased length of hospital stay, significant morbidity and mortality, and additional incurred costs for healthcare systems [1–4]. Studies have shown that ADRs can cause ≈5 % of hospital admissions, with a median bed stay of 8 days resulting in £466 million in additional annual costs to the UK National Health Service (NHS) [3, 4]. It has been shown that about half of ADR-related readmissions were deemed preventable, highlighting the importance of detecting and reporting side effects associated with medication use, to facilitate the early detection of ADRs [5–7].
Despite the emphasis on reporting of suspected ADRs, several studies showed low reporting rates [8–10]. In recognition of the possible contribution made by patients in the improvement of the detection of suspected ADRs, the YC Scheme was expanded to allow patients to report ADRs directly to the MHRA in February 2008 [11]. Analysis of patients’ reporting of ADRs demonstrated that patients’ reports are of value in identifying medicine-related ADRs, including serious ADRs [12], proving the importance of initiatives to increase rates of patient reporting. In Scotland, a 6-week campaign was conducted in 2008 with the purpose of encouraging patient reporting of ADRs via the YC Scheme; this campaign was shown to be effective [13]. Despite these initiatives, a survey in 2009 identified that fewer than 9 % of UK patients were aware of the YC Scheme [14]. Consequently, a second 6-week follow-up campaign was launched in Scotland in 2011 promoting patient reporting of ADRs to the YC Scheme, with an emphasis on herbal remedies. This subject was chosen to herald the European Traditional Medicinal Products Directive being implemented in April 2011. The objective of this study was to assess patterns in reporting of ADRs via the YC Scheme following the Scottish community pharmacy patient YC promotional campaign (January–February 2011).
Methods
The project was assessed by the South East Scotland Research Ethics Service and, as it involved investigating data that were anonymised to researchers and routinely collected as part of normal care, there was no need for an NHS ethical review (letter reference: NR/1402AB27). The project was approved by the Independent Scientific Advisory Committee for MHRA database research (ISAC).
Settings and study period
The study assessed YC reports for Scotland (≈5,295,400 inhabitants) received by YC Centre Scotland, and was compared with data from the nearest geographically located YC Centre region: Northern and Yorkshire (Yorkshire and the Humber plus the North East Strategic Health Authorities, but excluding Cumbria as these data were not available), with ≈8,266,000 inhabitants (i.e. the standard used the YC Centre comparator with the nearest similar healthcare characteristics and size). Data were received from the MHRA for the study period of January 2009–February 2012.
Study design and the intervention
All data were retrospectively collated on a weekly and monthly basis and duplication in reporting ADRs was removed (i.e. each YC was counted only once; data were normalised per 10,000 inhabitants). Overall, reporters were classified as (1) the patient group, including patients, parents and carers; (2) the HCP group, including all HCPs except community pharmacists; and (3) community pharmacists as a separate group from HCPs.
The mean weekly reporting rate was calculated for two scenarios:
-
1.
The pre-campaign period (January 2010–December 2010), during the 6-week campaign (3 January 2011–13 February 2011), and post-campaign (14 February 2011–28 February 2012).
-
2.
Only the 6 weeks during the campaign and the same 6-week periods in the pre- and post-campaign years. The rational for this was to determine if there were any differences between the full year and the same 6-week period in the winter period.
The intervention (the community pharmacy campaign) to promote patient reporting of ADRs via the YC Scheme, with an emphasis on herbal preparations, took place in Scotland (3 January 2011–13 February 2011). The Scottish campaign involved the following: posters were developed by YC Centre Scotland and patient leaflets provided by the MHRA to both raise awareness of potential side effects of herbal medicines and promote patient reporting of suspected ADRs to any medications via the patient YC Scheme [15, 16]. YC Centre Scotland developed an information sheet for community pharmacists, providing a background on the risk of ADRs with herbal medicines and illustrating how to encourage appropriate patient YC reporting. These promotional materials were distributed as part of the Scottish Government Public Health Service Poster Campaign. For the purposes of evaluating this campaign, the herbal medicines were defined as either products of plant origin or other unlicensed supplements listed in the Natural Medicines Comprehensive Database [17]. Approximately 1200 community pharmacies throughout Scotland received remuneration in return for participation in the public health campaign, which involved displaying the poster and leaflets in their pharmacies and encouraging them to answer customers’ queries and provide clarifications regarding the YC Scheme.
No other similar campaigns were conducted across the UK. However, at this time in Northern and Yorkshire and the rest of the UK (excluding Scotland), ≈20 % of general practitioners (GPs) started to use upgraded software (named SystmOne), which enabled them to submit electronic YCs to the MHRA in a quick and secure manner while updating their patient notes. This version of SystmOne was first introduced in November 2010; however, numbers of reports received were initially very low [18]. For the purposes of this study, January 2011 was considered the starting point for the assessment of the impact of SystmOne on GPs’ ADR reporting. Due to the introduction of SystmOne in Northern and Yorkshire, GP data were excluded and modelled separately using the time-series analysis methods [19]. The percentage of reported serious ADRs in Scotland, per different reporter groups, out of total serious ADRs was calculated as follows: [number of reported serious ADRs (by a specific group)/number of total reported serious ADRs (by all groups)] × 100. Serious ADRs were defined as anything life-threatening, disabling or incapacitating; anything resulting in or prolonging hospitalisation; congenital abnormalities; and anything deemed medically significant or resulting in death.
Statistical analysis
The impact of the Scottish campaign and the confounding unexpected effect of SystmOne’s introduction in the comparator YC Centre region were evaluated utilising the analysis of segmented regression of an interrupted time series as follows: monthly reported ADRs were modelled as reporting rates. Data were coded as described elsewhere (0 before campaign; 1 post campaign) [16]. An additional dummy variable (0, 1) was introduced in the model to take into account the effect of the sharp increase observed in November 2009, which resulted from a planned vaccination programme. A p value of <0.05 was considered to be statistically significant. The time-series analyses were performed using EViews 6 software (QMS, Irvine, CA, USA).
Results
During the study period (January 2009–February 2012), a total of 3610 non-duplicated YCs were submitted from Scotland. The mean monthly number of YCs submitted for the years 2009, 2010, and 2011 was 0.241, 0.174, and 0.144 per 10,000 inhabitants, respectively, indicating that the campaign had no impact on overall YC submissions.
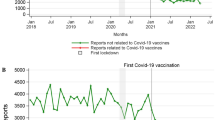
The mean weekly patient-reported ADRs before, during, and after the campaign in Scotland were 0.005, 0.002, and 0.004 per 10,000 inhabitants, respectively (Fig. 1a), again indicating that the campaign made no impact on YC reporting. The analysis of data showed no statistically significant level change (p = 0.983) and no significant trend change (p = 0.591) in YC reporting by patients.
Mean weekly submitted Yellow Cards before, during, and after the Scottish campaign per 10,000 inhabitants in Scotland and in Northern and Yorkshire, a comparing the 6-week campaign with 1 year before and 1 year after, and b comparing the 6-week campaign with the same 6-week period each year, January 2010–February 2012
Scottish community pharmacist reporting demonstrated a decreasing trend (i.e. pre-campaign = 0.002, during campaign = 0.002, post-campaign = 0.001 per 10,000 inhabitants) [Fig. 1a]. When comparing the 6-week campaign of community pharmacist ADR reporting with the same period in the year before and the year after the campaign in Scotland, an increase in the ADR reporting rate was observed during the campaign, followed by a decrease in the post-campaign period (Fig. 1b). The campaign showed no impact on level change (p = 0.166) nor on trend change (p = 0.404) among Scottish community pharmacists reporting ADRs.
The mean weekly reported ADRs (excluding GPs’ reports) before, during, and after the campaign in Scotland were 0.029, 0.019, and 0.023 per 10,000 inhabitants, respectively. The analysis of time series for monthly reported YCs in Scotland (excluding GPs’ reports) demonstrated no statistically significant level change and no significant trend change post-campaign (p = 0.706 and p = 0.509, respectively).
Relative to before the campaign, mean weekly GP reporting of ADRs was lower during and after the campaign (Fig. 1a). Slight increase in GP reporting of ADRs was observed when comparing the 6-week campaign with the same period in the years before and after the campaign (Fig. 1b); however, the campaign had no impact on the level or trend changes in GPs reporting ADRs in Scotland.
Although GP ADR reporting decreased in Scotland following the Scottish public health campaign, YC reporting increased in Northern and Yorkshire after the introduction of SystmOne in January 2011 (Fig. 2c) and was associated with a statistically significant (p = 0.001) impact on improving GP reporting of ADRs (i.e. 0.036 ADR reports per 10,000 inhabitants/month). For a complete comparison with comparator Northern and Yorkshire, the graph for the monthly series for patients’, community pharmacists’, and GPs’ YC reporting in Scotland versus Northern and Yorkshire can be seen in Fig. 2.
Overall, the number of reported herbal medicines in Scotland was low (eight reports between January 2010 and November 2011). Further analysis was not possible because of the small number of reports. The percentages for serious ADR reporting before and during the campaign were similar (51 %) in Scotland, but an increase in reporting was observed following the campaign (56 %). Evaluation of monthly reporting of serious ADRs per 10,000 inhabitants in Scotland shows a non-significant change in the level (p = 0.649) and trend (p = 0.579). Percentages for reported serious ADRs, as total of serious reported YCs, for different reporting groups in Scotland (Fig. 3) show variations in reporting among the groups (i.e. GPs, community pharmacists, and patients), but little change within each group itself. Overall the percentage for reporting serious ADRs for community pharmacists was much lower than for the other groups (i.e. <5 % vs. range 20–33 %; Fig. 3).
The medicines and ADRs reported were shown to vary for the periods before, during, and after the campaign in Scotland for both patients and HCPs (Table 1). The most highly reported drugs pre-/post-campaign by the patient group were influenza vaccine (15.9 %) and varenicline (5.4 %), respectively. Several drugs were reported by the patient group at a similar frequency during the campaign (Table 1). For HCPs, the influenza vaccine, diphtheria-containing vaccines, and varenicline were reported in all three periods (Table 1). Regarding the suspected ADRs reported by the patient group, nausea (2.8 %) was the most frequently reported ADR pre-campaign, whereas dizziness (2.2 %) was the most highly reported ADR post-campaign. However, several ADRs were reported by the patient group at a similar frequency pre- and post-campaign (Table 2). Nausea (3.3 %) was the highest reported ADR by health professionals pre-campaign, while tachycardia or injection site swelling (both 2.2 %) and headache (2.1 %) were the highly reported ADRs during the campaign and post-campaign (Table 2).
In the assessment of the quality of reports received from patients, comparing percentages of missing fields from the YC reports between the 2008 and the 2011 Scottish campaigns, there was observable improvement in reporting for the quality indicators reaction outcome, patient age, patient initials, patient weight and height, and route of administration (Table 3).
Discussion
The continued development and marketing of new medicines along with the increased complexity of patients’ medicines regimens (i.e. polypharmacy) has underpinned the need to create efficient systems to detect and prevent the development of ADRs. In addition to HCPs’ ADR reports, those submitted by patients add value by giving details on the impact upon the quality of their life that is not provided by HCPs, and contribute to the generation of new safety signals [12]. As community pharmacies are well distributed geographically and are easily accessible by patients, they can play an important role in promoting the reporting of suspected ADRs to patients [20]. The results of this study, however, showed that this public health campaign via community pharmacies across Scotland had no impact on influencing the overall YC reporting of ADRs. No significant level change and no significant trend change in overall, patient and community pharmacist ADR reporting post-campaign were observed. When comparing the 6-week campaign with the same 6-week period in each year before and after the campaign, a slight increase in community pharmacists’ YC reporting was observed during the campaign, followed by a slight decrease post-campaign. However, numbers of submitted reports were very small and the reporting rate for community pharmacists was much lower than for GPs. Similar findings were observed when the average weekly ADR submissions were evaluated for the other groups besides the community pharmacist group.
No patterns with medicines or suspected ADRs reported before, during, or after the campaign were discernible. Because of the very low reporting numbers involved for herbal medicines, it was not possible to analyse these to draw any conclusions.
Although the present Scottish campaign had no impact on increasing patients’ reporting of ADRs, other similar promotional activities have been more successful [13, 21–23]. Reasons for the poor outcome could include several factors. The timing of the implementation of the campaign coincided with very low temperatures in December 2010, with snow continuing to lie into early January 2011 [24, 25]. The very cold weather and snow in December continued into January, and it was considered that the treacherous conditions would have discouraged people from going outdoors to visit their pharmacy and, therefore, they would not have been exposed to the promotional poster at the beginning of the campaign. In particular, it is possible that elderly people, who tend to be the greatest users of medicines and be at the greatest risk of side effects, would have been less likely to visit a pharmacy during this time. In addition, although the campaign incorporated the reporting of ADRs in general, the key message focused on reporting ADRs with herbal medicine products, which may have been too narrow a subject [15].
Whereas no significant changes in monthly GPs’ ADR reporting were observed in Scotland, a significant increase in their reporting levels was observed in the comparator region of Northern and Yorkshire following the implementation of an integrated electronic Yellow Card (eYC) into the SystmOne practice software. This software allows easier and faster reporting of ADRs to the MHRA by facilitating the population of eYCs directly from the GPs’ computers [18]. A subsequent report has confirmed that this system has contributed to an increase in the number of reports received from GPs, accounting for 63 % of GPs’ reports in 2012 [18].
The importance of reporting serious ADRs is emphasised by the MHRA, which requests that serious ADRs be reported for all medicines. The percentages for reporting serious ADRs before and during the campaign were similar in Scotland; an increase in reporting percentages was observed following the campaign. The latter increase was not statistically significant; however, this may correlate with a dilution in reporting of ADRs associated with medicines with a Black Triangle (BT) status, for which all ADRs should be reported. Drugs undergoing intensive monitoring by the regulatory authority are allocated BT Status. All new medicines have BT status for at least the first 5 years after they are marketed.
It is interesting to note that patients reported more ADRs than community pharmacists. The low reporting rate of community pharmacists was also observed in the 2008 Scottish campaign [13] and in other reports [26, 27]. It is possible that the community pharmacists were encouraging patients to report suspected ADRs themselves rather than reporting them on their behalf. Further research is needed to explore this proposed possible confounding factor. Promotion of patient reporting of suspected ADRs via the YC by community pharmacists is an important professional responsibility to empower patients and to ensure the continued utility of the YC Scheme as the backbone of pharmacovigilance in the UK.
Studies showed that comparing patients’ ADR reports with those of HCPs might generate different information in terms of suspected medicines and reactions to medicines [28]. For example, in the post-campaign period, patients and HCPs reported similar suspected medicines (e.g. varenicline, human papillomavirus vaccine), but patients were more likely than HCPs to report ADRs with other medicines (e.g. citalopram, diclofenac), and less likely to report other suspected ADRs with medicines more frequently reported by HCPs (e.g. adalimumab). Of note, more ADR reports for BT medicines were observed among HCPs’ reports than among patients’ reports, which indicates that HCPs are possibly more vigilant with reporting of suspected ADRs to newer medicines that have less safety experience available post-marketing. Varenicline was the top reported BT medicine for both patients and HCPs. This might be related to the intensive monitoring and publicity this medicine has received and patient education with close follow-up, which may have increased the awareness of both patients and HCPs [29, 30].
The results of the assessment of the quality of reported data highlighted variations in completeness of records between patients in Scotland (2008 vs. 2011 campaign). The completeness of these fields is important for including essential details regarding the reported suspected ADRs and the signal identification capacity. It is interesting to note that the recording of certain fields by the patient group improved in the 2011 campaign relative to the 2008 campaign. One possible explanation for this could be the increased usage of the eYC website, as submitting an electronic record is not possible without the completion of certain fields such as outcome [12, 18]. Nevertheless, comparing data completeness in the 2011 campaign with the 2008 campaign [13] showed a decrease in recording percentages for some fields in the 2011 campaign (e.g. patients’ recording of dose and indication). Further assessment and insight into the possibility of improving the completeness of the latter fields by modifying the eYC is needed.
The strengths of this study include:
-
Its contribution to the growing knowledge in this area, as few studies involving community pharmacies promotion of patients’ reporting of ADRs via YC have been conducted.
-
Its evaluation of the change in ADR reporting rates using historical comparisons and comparisons with the nearest geographically located region (Northern and Yorkshire).
-
Its use of a robust statistical method (i.e. segmented regression of an interrupted time series, to assess the impact of the promotional campaign on ADR reporting).
-
Its use of data routinely collected by the MHRA through an established reporting system, which minimises selection and information biases.
The study’s limitations include:
-
The implementation of the promotional campaign only in Scotland, which may limit the generalisability of the findings to other regions in the UK or elsewhere.
-
The weather conditions at the time of the campaign (i.e. one of the worst winters in the last decade was observed during the campaign period), which may have influence the results and their interpretation.
-
The very limited number of reports for some of the assessed outcomes (e.g. herbal medicines), making it impossible to conduct appropriate statistics and provide definitive conclusions; the study would have benefitted from a larger sample size, but this was not possible.
-
The need for additional qualitative research aimed at providing an in-depth assessment of the content of the reported variables in the assessment of the quality of patients’ completeness of records between the Scottish 2008 and 2011 promotional campaigns.
In conclusion, the results of this study showed that this 2011 public health campaign had no impact on influencing the overall submission of YCs. This study adds to a growing body of required information in this area; the cost-effectiveness of such efforts requires further research. The resultant recommendations from this study are as follows: (1) if future promotional campaigns are being considered, if possible they should be scheduled for a time when the normal predicted weather would not restrict patients’ movements, possibly springtime; and qualitative attitudinal data should be collected and evaluated to help evaluate the effectiveness of any such campaign; (2) all distributed educational material and promotional activity should adequately emphasise the need to report suspected ADRs as a general concept; and (3) increasing community pharmacists’ knowledge of ADRs, through educational materials such as the NHS Education for Scotland/YC Centre Scotland ADR eLearning Modules [31], might aid pharmacists in being more actively involved in both reporting ADRs themselves and promoting patient ADRs reporting in normal routine day-to-day practice.
References
Classen DC, Pestotnik SL, Evans RS, et al. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA. 1997;277(4):301–6.
Rodríguez-Monguió R, Otero MJ, Rovira J. Assessing the economic impact of adverse drug effects. Pharmacoeconomics. 2003;21(9):623–50.
Kongkaew C, Noyce PR, Ashcroft DM. Hospital admissions associated with adverse drug reactions: a systematic review of prospective observational studies. Ann Pharmacother. 2008;42(7):1017–25.
Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18,820 patients. BMJ. 2004;329(7456):15–9.
Davies EC, Green CF, Mottram DR, et al. Emergency re-admissions to hospital due to adverse drug reactions within 1 year of the index admission. Br J Clin Pharmacol. 2010;70(5):749–55.
Winterstein AG, Hatton RC, Gonzalez-Rothi R, et al. Identifying clinically significant preventable adverse drug events through a hospital’s database of adverse drug reaction reports. Am J Health Syst Pharm. 2002;59(18):1742–9.
Thomsen LA, Winterstein AG, Søndergaard B, et al. Systematic review of the incidence and characteristics of preventable adverse drug events in ambulatory care. Ann Pharmacother. 2007;41(9):1411–26.
Heeley E, Riley J, Layton D, et al. Prescription-event monitoring and reporting of adverse drug reactions. Lancet. 2001;358(9296):1872–3.
Martin RM, Kapoor KV, Wilton LV, et al. Underreporting of suspected adverse drug reactions to newly marketed (“black triangle”) drugs in general practice: observational study. BMJ. 1998;317(7151):119–20.
Hazell L, Shakir SA. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29(5):385–96.
Hazell L, Cornelius V, Hannaford P, et al. How do patients contribute to signal detection? A retrospective analysis of spontaneous reporting of adverse drug reactions in the UK’s Yellow Card Scheme. Drug Saf. 2013;36(3):199–206.
Avery AJ, Anderson C, Bond CM, et al. Evaluation of patient reporting of adverse drug reactions to the UK ‘Yellow Card Scheme’: literature review, descriptive and qualitative analyses, and questionnaire surveys. Health Technol Assess. 2011;15(20):1–234.
Kitto L. Patient reporting of adverse drug reactions: a quantitative study. 2009. http://www.yccscotland.scot.nhs.uk/publications/Pages/Journal-Articles-regarding-Adverse-Drug-Reactions.aspx. Accessed 27 Dec 2014.
Fortnum H, Lee AJ, Rupnik B, et al. Survey to assess public awareness of patient reporting of adverse drug reactions in Great Britain. J Clin Pharm Ther. 2012;37(2):161–5.
Yellow Card Centre Scotland. Training posters. http://www.yccscotland.scot.nhs.uk/training/Pages/Posters.aspx. Accessed 6 July 2014.
MHRA-Yellow Card. Patient information cards 2010. https://yellowcard.mhra.gov.uk/_assets/files/Member-of-Public-Information-Card.pdf. Accessed 6 July 2014.
Natural Medicines Comprehensive Database. http://naturaldatabase.therapeuticresearch.com/home.aspx?cs=&s=ND&AspxAutoDetectCookieSupport=1. Accessed 6 July 2014.
MHRA. Trends in UK spontaneous Adverse Drug Reaction (ADR) reporting between 2008–2012. http://www.mhra.gov.uk/home/groups/plp/documents/websiteresources/con408250.pdf. Accessed 10 Aug 2014.
Wagner AK, Soumerai SB, Zhang F, et al. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299–309.
Planas LG, Kimberlin CL, Segal R, et al. A pharmacist model of perceived responsibility for drug therapy outcomes. Soc Sci Med. 2005;60(10):2393–403.
Van Hunsel F, Passier A, van Grootheest K. Comparing patients’ and healthcare professionals’ ADR reports after media attention: the broadcast of a Dutch television programme about the benefits and risks of statins as an example. Br J Clin Pharmacol. 2009;67(5):558–64.
Martin RM, May M, Gunnell D. Did intense adverse media publicity impact on prescribing of paroxetine and the notification of suspected adverse drug reactions? Analysis of routine databases, 2001–2004. Br J Clin Pharmacol. 2006;61(2):224–8.
Leone R, Moretti U, D’Incau P, et al. Effect of pharmacist involvement on patient reporting of adverse drug reactions: first Italian study. Drug Saf. 2013;36(4):267–76.
The Met Office is the UK’s National Weather Service, 2011. http://www.metoffice.gov.uk/climate/uk/2011/january.html. Accessed 4 Aug 2014.
http://www.bbc.co.uk/news/uk-scotland-12141718. Accessed 4 Aug 2014.
Gedde-Dahl A, Harg P, Stenberg-Nilsen H, et al. Characteristics and quality of adverse drug reaction reports by pharmacists in Norway. Pharmacoepidemiol Drug Saf. 2007;16(9):999–1005.
van Grootheest AC, de Jong-van den Berg LT. The role of hospital and community pharmacists in pharmacovigilance. Res Social Adm Pharm. 2005;1(1):126–33.
Inch J, Watson MC, Anakwe-Umeh S. Patient versus healthcare professional spontaneous adverse drug reaction reporting: a systematic review. Drug Saf. 2012;35(10):807–18.
Lasser KE, Boyd JW. Varenicline and smokers with mental illnesses. Lancet. 2008;372(9645):1218–9.
Purvis TL, Nelson LA, Mambourg SE. Varenicline use in patients with mental illness: an update of the evidence. Expert Opin Drug Saf. 2010;9(3):471–82.
Education for Scotland. Educational resources—patient safety: adverse drug reactions. Modules 1–6; http://www.nes.scot.nhs.uk/education-and-training/by-discipline/pharmacy/about-nes-pharmacy/educational-resources/resources-by-topic/clinical-governance/patient-safety-adverse-drug-reactions.aspx. Accessed 10 Aug 2014.
Acknowledgments
We would like to thank Ms. Sharon Suri (MHRA) for assistance in extraction of the YC data, and Dr. Alistair Millar (NHS Lothian) and Mrs. Anne MacKay (YC Centre Scotland) for their help with dataset manipulation. We are also indebted to Mrs. Moira Kinnear (NHS Lothian) for facilitating liaison with the University of Strathclyde, and to Ms. Sarah Smith (YC Centre Northern and Yorkshire) for assistance with clarification of the contents of their dataset.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to conduct the study or prepare this report.
Conflicts of interest
M. A. Aldeyab S. C. Noble, M. Cuthbert, S. Maxwell, J. Dear, and A. Boyter declare that they have no conflicts of interest relevant to the content of this manuscript.
Ethical approval
Because of the study design, NHS ethical review was not required. The project was approved by ISAC.
Rights and permissions
About this article
Cite this article
Aldeyab, M.A., Noble, S.C., Cuthbert, M. et al. Assessment of the impact of the Scottish public health campaign on patient reporting of adverse drug reactions. Drugs Ther Perspect 32, 209–218 (2016). https://doi.org/10.1007/s40267-016-0280-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-016-0280-y