Abstract
Background
Programmed death-ligand 1 (PD-L1) inhibitor plus platinum–etoposide chemotherapy is used as a first-line treatment for extensive-stage small cell lung cancer (ES-SCLC), regardless of age.
Objective
We examined the role of the Geriatric 8 (G8) screening tool for evaluating treatment outcomes in patients with ES-SCLC treated with PD-L1 inhibitor plus platinum–etoposide chemotherapy as first-line therapy.
Patients and Methods
Between September 2019 and October 2021, we prospectively evaluated patients with ES-SCLC treated with immunochemotherapy at ten institutions in Japan. The G8 score was assessed before treatment initiation.
Results
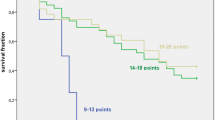
We evaluated 44 patients with ES-SCLC. Patients with G8 score > 11 had longer overall survival (OS) than those with G8 score ≤ 11 (not reached versus 8.3 months; log-rank test, p = 0.005). In univariate and multivariate analyses, G8 score > 11 [hazard ratio (HR) 0.34; 95% confidence interval (CI) 0.15–0.75; p = 0.008 and HR 0.34; 95% CI 0.14–0.82; p = 0.02, respectively) and performance status (PS) of 2 (HR 5.42; 95% CI 2.08–14.2; p < 0.001 and HR 6.94; 95% CI 2.25–21.4; p < 0.001, respectively) were independent prognostic factors for OS. Among patients with good PS (0 or 1), the OS in patients with G8 score > 11 was significantly longer than that in patients with G8 score ≤ 11 (not reached versus 12.3 months; log-rank test, p = 0.02).
Conclusions
G8 score evaluation before treatment initiation was useful as a prognostic factor for ES-SCLC patients who received PD-L1 inhibitors and platinum–etoposide chemotherapy, even with good PS.



Similar content being viewed by others
References
Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24:4539–44. https://doi.org/10.1200/JCO.2005.04.4859.
Gazdar AF, Bunn PA, Minna JD. Small-cell lung cancer: what we know, what we need to know and the path forward. Nat Rev Cancer. 2017;17:725–37. https://doi.org/10.1038/nrc.2017.87.
Farago AF, Keane FK. Current standards for clinical management of small cell lung cancer. Transl Lung Cancer Res. 2018;7:69–79. https://doi.org/10.21037/tlcr.2018.01.16.
Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer. 2015;121:664–72. https://doi.org/10.1002/cncr.29098.
Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379:2220–9. https://doi.org/10.1056/NEJMoa1809064.
Liu SV, Reck M, Mansfield AS, Mok T, Scherpereel A, Reinmuth N, et al. Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133). J Clin Oncol. 2021;39:619–30. https://doi.org/10.1200/JCO.20.01055.
Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (Caspian): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394:1929–39. https://doi.org/10.1016/S0140-6736(19)32222-6.
Pallis AG, Shepherd FA, Lacombe D, Gridelli C. Treatment of small-cell lung cancer in elderly patients. Cancer. 2010;116:1192–200. https://doi.org/10.1002/cncr.24833.
Shiono A, Imai H, Wasamoto S, Tsuda T, Nagai Y, Minemura H, et al. Real-world data of atezolizumab plus carboplatin and etoposide in elderly patients with extensive-disease small-cell lung cancer. Cancer Med. 2023;12:73–83. https://doi.org/10.1002/cam4.4938.
Morimoto K, Yamada T, Takeda T, Shiotsu S, Date K, Harada T, et al. Efficacy and safety of programmed death-ligand 1 inhibitor plus platinum-etoposide chemotherapy in patients with extensive-stage SCLC: a prospective observational study. JTO Clin Res Rep. 2022;3:100353. https://doi.org/10.1016/j.jtocrr.2022.100353.
Morimoto K, Yamada T, Yokoi T, Kijima T, Goto Y, Nakao A, et al. Clinical impact of pembrolizumab combined with chemotherapy in elderly patients with advanced non-small-cell lung cancer. Lung Cancer. 2021;161:26–33. https://doi.org/10.1016/j.lungcan.2021.08.015.
Wildiers H, Heeren P, Puts M, Topinkova E, Janssen-Heijnen ML, Extermann M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32:2595–603. https://doi.org/10.1200/JCO.2013.54.8347.
Bellera CA, Rainfray M, Mathoulin-Pélissier S, Mertens C, Delva F, Fonck M, et al. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol. 2012;23:2166–72. https://doi.org/10.1093/annonc/mdr587.
Takahashi M, Takahashi M, Komine K, Yamada H, Kasahara Y, Chikamatsu S, et al. The G8 screening tool enhances prognostic value to ECOG performance status in elderly cancer patients: a retrospective, single institutional study. PLoS ONE. 2017;12:e0179694. https://doi.org/10.1371/journal.pone.0179694.
Jespersen E, Winther SB, Minet LR, Möller S, Pfeiffer P. Frailty screening for predicting rapid functional decline, rapid progressive disease, and shorter overall survival in older patients with gastrointestinal cancer receiving palliative chemotherapy—a prospective, clinical study. J Geriatr Oncol. 2021;12:578–84. https://doi.org/10.1016/j.jgo.2020.10.007.
van Walree IC, Scheepers E, van Huis-Tanja L, Emmelot-Vonk MH, Bellera C, Soubeyran P, et al. A systematic review on the association of the G8 with geriatric assessment, prognosis and course of treatment in older patients with cancer. J Geriatr Oncol. 2019;10:847–58. https://doi.org/10.1016/j.jgo.2019.04.016.
Morimoto K, Uchino J, Yokoi T, Kijima T, Goto Y, Nakao A, et al. Impact of cancer cachexia on the therapeutic outcome of combined chemoimmunotherapy in patients with non-small cell lung cancer: a retrospective study. Oncoimmunology. 2021;10:1950411. https://doi.org/10.1080/2162402X.2021.1950411.
Shiotsu S, Yoshimura A, Yamada T, Morimoto K, Tsuchiya M, Yoshioka H, et al. Pembrolizumab monotherapy for untreated PD-L1-Positive non-small cell lung cancer in the elderly or those with poor performance status: a prospective observational study. Front Oncol. 2022;12:904644. https://doi.org/10.3389/fonc.2022.904644.
Youn B, Trikalinos NA, Mor V, Wilson IB, Dahabreh IJ. Real-world use and survival outcomes of immune checkpoint inhibitors in older adults with non-small cell lung cancer. Cancer. 2020;126:978–85. https://doi.org/10.1002/cncr.32624.
Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–92. https://doi.org/10.1056/NEJMoa1801005.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8. https://doi.org/10.1038/bmt.2012.244.
Agemi Y, Shimokawa T, Sasaki J, Miyazaki K, Misumi Y, Sato A, et al. Prospective evaluation of the G8 screening tool for prognostication of survival in elderly patients with lung cancer: a single-institution study. PLoS ONE. 2019;14:e0210499. https://doi.org/10.1371/journal.pone.0210499.
Schulkes KJG, Souwer ETD, van Elden LJR, Codrington H, van der Sar-van der Brugge S, Lammers JJ, et al. Prognostic value of geriatric 8 and identification of seniors at risk for hospitalized patients screening tools for patients with lung cancer. Clin Lung Cancer. 2017;18:660-666.e1. https://doi.org/10.1016/j.cllc.2017.02.006.
Couderc AL, Tomasini P, Nouguerède E, Rey D, Correard F, Montegut C, et al. Older patients treated for lung and thoracic cancers: unplanned hospitalizations and overall survival. Clin Lung Cancer. 2021;22:e405–14. https://doi.org/10.1016/j.cllc.2020.06.004.
Howlader N, Forjaz G, Mooradian MJ, Meza R, Kong CY, Cronin KA, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383:640–9. https://doi.org/10.1056/NEJMoa1916623.
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–95. https://doi.org/10.1016/S1470-2045(10)70218-7.
Friedlaender A, Liu SV, Passaro A, Metro G, Banna G, Addeo A. The role of performance status in small-cell lung cancer in the era of immune checkpoint inhibitors. Clin Lung Cancer. 2020;21:e539–43. https://doi.org/10.1016/j.cllc.2020.04.006.
Masubuchi K, Imai H, Wasamoto S, Tsuda T, Minemura H, Nagai Y, et al. Post-progression survival after atezolizumab plus carboplatin and etoposide as first-line chemotherapy in small cell lung cancer has a significant impact on overall survival. Thorac Cancer. 2022;13:2776–85. https://doi.org/10.1111/1759-7714.14621.
Acknowledgments
We thank the patients, their families, and all the investigators involved in this study. Additionally, we thank Editage (www.editage.jp) for their help with the English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Not applicable.
Conflicts of Interest
Tadaaki Y received grants from Pfizer, Ono Pharmaceutical, Janssen Pharmaceutical, and Takeda Pharmaceutical and personal fees from Eli Lilly. KT received grants from Chugai Pharmaceutical and Ono Pharmaceutical, and personal fees from AstraZeneca, Chugai Pharmaceutical, MSD, Eli Lilly, Boehringer Ingelheim, and Daiichi Sankyo. The other authors have no conflicts of interest to declare.
Ethical Approval
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and the study protocol was approved by the institutional review board of Kyoto Prefectural University of Medicine (ERB-C-1580), and each participating hospital and was registered at the University Medical Hospital Information Network (UMIN) Clinical Trials Registry (UMIN000044048).
Consent to Participate
All patients provided written informed consent.
Consent for Publication
Not applicable
Data Availability Statement
The datasets generated during the current study are not publicly available because of ethical constraints but are available from the corresponding author upon reasonable request.
Code Availability
Not applicable
Authors Contributions
KM and Tadaaki Y contributed to the study conception and design. KM, TT, SS, KD, TH, NT, YC, YT, Takahiro Y, HK, and Masaki I obtained the clinical data. Data were interpreted by KM, Tadaaki Y, Masaki I, AY, Masahiro I, ST, YHK, and KT. The manuscript was prepared by KM, Tadaaki Y, and KT. The final version of the manuscript was read and approved by all the authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Morimoto, K., Yamada, T., Takeda, T. et al. Prospective Observational Study Evaluating the Prognostic Value of the G8 Screening Tool for Extensive-Stage Small Cell Lung Cancer Patients Who Received Programmed Death-Ligand 1 Inhibitor plus Platinum–Etoposide Chemotherapy. Drugs Aging 40, 563–571 (2023). https://doi.org/10.1007/s40266-023-01034-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-023-01034-4