Abstract
Introduction
Frailty is associated with an increased risk of death and morbid events. Frail individuals are known to have multiple comorbidities which are often associated with polypharmacy. Whilst a relationship between polypharmacy and frailty has been demonstrated, it is not clear if there is an independent relationship between frailty and medication harm.
Aims
This scoping review aimed to identify and critically appraise studies evaluating medication harm in patients with frailty.
Methods
PubMed, EMBASE, CINAHL and Cochrane databases were searched from inception until 1 February 2021 using key search terms that are synonymous with frailty (such as frail and frail elderly) and medication harm (such as adverse drug events and adverse drug reactions). To be included, studies must have identified medication harm as a primary or secondary outcome measure, and used a frailty assessment tool to determine frailty, or clearly defined how frailty was assessed. Data were narratively synthesised and presented in tables. The checklist from the Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies from the National Heart, Lung, and Blood Institute was used to assess the quality and risk of bias of studies that met the inclusion criteria.
Results
Of 2685 retrieved abstracts, 24 underwent full-text review and nine studies met the inclusion criteria. Three studies were retrospective cohort studies, and six were prospective observational studies. Six studies comprised two distinct groups of frail and non-frail individuals, and the remaining three studies evaluated medication harm in an entirely frail population. Seven studies used validated frailty tools such as the Clinical Frailty Scale, Fried Frailty Index, and Fried Frailty Phenotype. Two studies measured frailty using self-defined criteria. Overall, frail individuals were at risk of medication harm with rates ranging between 18.7 and 77% across the nine studies. However, whether frailty is an independent predictor of medication harm remains uncertain, as this was only evaluated in one study. The risk of bias assessment identified limitations in methods and reporting with all nine studies.
Conclusion
This scoping review identified nine studies evaluating medication harm in frail patients. However, all were limited by the methodological quality and inadequate reporting of study factors. There are few high-quality studies that described a relationship between medication harm and frailty. More robust studies are required that examine the independent relationship between frailty and medication harm, after adjusting for all possible confounders and in particular polypharmacy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A variety of different methods are used to detect medication harm and to evaluate causality across studies. |
Studies assessing frailty as an independent predictor of medication harm are limited in number and of poor methodological quality. |
In general, frail individuals appear to be at high risk of medication harm and should be prioritised for medication review and optimisation. |
Frailty may be incorporated within risk prediction tools to help identify patients at high risk of medication harm for a timely and targeted medication review. |
1 Introduction
Medication harm and frailty have both been associated with adverse outcomes, including hospital admission, increased length of hospital stay, and mortality [1, 2]. Frailty is common in older individuals, affecting 26% of Australian adults aged 65 years and older, with a further 48% identified as pre-frail [3]. Internationally, the prevalence of frailty across all age groups has been reported as up to 14% [3]. Frail individuals often have multiple comorbidities [4]. In frail individuals, minor stresses may lead to a disproportionate decline in physical and mental well-being which may not recover to baseline [5]. The deficit accumulation model of frailty [6] shows that individuals with multiple health deficits are at increased risk for adverse outcomes such as falls, hospitalisation, and mortality [7, 8].
Fried et al. describe frailty as a clinical syndrome in which three or more of the following criteria are present: unintentional weight loss (10 pounds in the past year), self-reported exhaustion, weakness (grip strength), slow walking speed, and low physical activity. Rockwood et al. have defined frailty as deficit accumulation from the loss of functional reserves (energy, physical ability, cognition, health). Whilst old age is not synonymous with frailty, frailty syndromes are more common in older adults [9], with studies reporting up to 59% of older adults living in the community as being frail [10]. Given that frailty is associated with multiple adverse patient outcomes, a patient’s level of frailty should be considered in therapeutic decisions, particularly for older adults who are also often subject to polypharmacy, and who may be at an increased risk of medication harm [11]. Other potential factors include inappropriate prescribing and pharmacokinetic/pharmacodynamic changes associated with aging which may put individuals with frailty at risk of poorer patient outcomes [11].
Medication harm is an area of growing interest with clinicians and researchers who seek interventions to improve patient safety [12]. Medication harm is defined as “any negative patient outcome or injury, related to medication use, irrespective of severity or preventability” [13]. It is especially common in older adults [14], may result in hospitalisation, morbidity, and mortality, and has fiscal implications for the healthcare system [15]. It is estimated that medication harm accounts for 3.6% of all hospital admissions and occurs at an estimated rate of 10% during the inpatient stay [16]. These figures are likely to underestimate harm as there is frequent under reporting [16]. In the residential aged care setting, it is estimated that at least 14% of residents have one or more adverse drug reactions over a 12-month period [16]. Detection and classification of medication harm using causality and preventability tools have identified that up to 40% of medication harm events are preventable [17]. The World Health Organisation (WHO) has issued a global safety challenge to achieve a 50% reduction in preventable medication harm by 2022 [18].
Early identification of individuals at high risk of medication harm to whom preventive strategies are targeted can improve health outcomes and reduce healthcare costs [19]. Medication harm is especially common in older adults due to comorbidities, polypharmacy, and an age-related decline in the physiological ability to metabolise and eliminate medication [20]. If frailty is superimposed, we hypothesise that the risk of medication harm is increased, although, to the best of our knowledge, there has been no review of studies that have investigated this hypothesis. Therefore, we undertook a review of studies to determine if there is an association between frailty and medication harm.
2 Methods
This review was conducted and reported according to the Preferred Reporting Items for Systematic reviews and Meta-Analysis extension for Scoping Reviews (PRISMA-ScR) Guidelines [21]. The protocol had been registered with Open Science Framework (10.17605/OSF.IO/DMKQ4).
All articles generated from searches in the relevant databases were screened by title and abstract by two reviewers, JL and either NF or DL, to identify potentially suitable studies. Full-text review for the inclusion of the eligible studies was conducted by two reviewers (JL and NF or DL). Discrepancies were resolved through discussion and where necessary through advice from a third reviewer (MB).
Given the heterogeneous methods, results, and reporting of included studies, we chose to describe key findings narratively and categorise results in tables. The studies were grouped based on whether they reported a non-frail (also known as robust) control population, or whether they only included a frail population.
In addition to evaluating frailty and its association to medication harm, we also determined if the studies had assessed the causality, severity, and preventability using validated methods. Data extraction and reporting included the statistical analysis provided by each study such as p values, odds ratios, confidence intervals, risk ratios, and hazards ratios.
2.1 Inclusion Criteria
Medication harm refers to any harm or injury relating to medication use [13]. As a clear description of medication harm across some studies is lacking, we included medication harm and related synonyms such as adverse drug reactions and adverse drug events.
Studies were considered for inclusion if they stated medication harm as a primary and/or secondary outcome. Studies were also included if they appraised other secondary measures (inappropriate prescribing, medication error, and non-adherence).
Studies must have evaluated medication harm in an exclusively frail population, either as a subset of a larger population or as a cohort in which all study participants were deemed to be frail. To be included, frailty should have been clearly defined (using either tools or criteria as defined in existing literature). There were no restrictions to the study design.
2.2 Exclusion Criteria
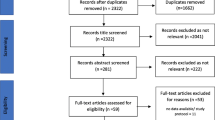
Studies were excluded if medication harm was not reported as their primary or secondary outcome (Fig. 1), and if there was no clearly defined method for assessing frailty. Publications in languages other than English were excluded.
2.3 Information Sources and Search Strategy
A scoping literature search was undertaken in four databases from the time of database inception until 1 December 2021. The databases included PubMed, Embase, Cumulative Index to Nursing and Allied Health (CINAHL), and the Cochrane Library. Studies were identified using medical subject headings (MeSH) and keywords in the title or abstract related to medication harm and frailty. Medication harm was denoted by adverse drug event (ADE), adverse drug reaction (ADR), medication misadventure, drug-related harm, and medication-related harm. Frailty was denoted by terms within commonly used validated frailty assessment tools such as Clinical Frailty Scale (CFS)/Rockwood Frailty Score, Edmonton Frail Scale, and Fried’s Frailty Index (FFI), combined with other words synonymous with frailty such as frail, frail elderly, frail syndrome using the Boolean operator ‘AND.’ The full search strategies for each database are included in the electronic supplementary materials (ESM; Appendix I). Google Scholar and citation searching from citations and references lists of the included studies were also undertaken to identify additional studies.
2.4 Definition of Frailty
In general, there are two broad models of frailty, which are the phenotype model [22] and the cumulative deficit model [23]. For the phenotype model [22], individuals are assessed through selected patient characteristics (e.g., unintentional weight loss, reduced muscle strength, reduced gait speed, self-reported exhaustion, and low energy expenditure); if those characteristics are present, poorer outcomes could be predicted. Individuals with three or more characteristics are considered to be frail, and others with lesser characteristics could be identified as pre-frail or robust. However, in the cumulative deficit model, individuals will accumulate deficits, including signs/symptoms/disease (e.g., loss of hearing, low mood, tremor, dementia) [6]. These deficits add to the increase in frailty index [24].
2.5 Definition of Medication Harm
Common terms used synonymously in the literature to describe medication harm include adverse drug events (ADE), adverse drug reactions (ADR), and drug-related problems (DRPs). Terms are often used based on researcher preference and their definition can vary, in that some studies include all actual patient harm related to the use of medicine whilst others include medication errors. The different methods/definitions of each study are shown in Table 2.
ADE and ADR are commonly defined by the International Classification of Diseases (ICD-10) codes. World Health Organisation (WHO) [25] provides a clear definition of ADR as “any response to a drug which is noxious and unintended, and which occurs at doses normally used in man for prophylaxis, diagnosis, or therapy of disease, or for the modification of physiological function.” The following definition of ADR by Edwards and Aronson [26] was adopted: "an appreciably harmful or unpleasant reaction, resulting from an intervention related to the use of a medicinal product, which predicts hazard from future administration and warrants prevention or specific treatment, or alteration of the dosage regimen, or withdrawal of the product." Chemotherapy-related toxicity is defined using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) [27].
2.6 Assessment of Medication Harm
As medication harm is a major healthcare problem [28, 29], greater emphasis is needed to correctly identify and evaluate events. This is crucial to accurately determine harm resultant from a medication. Only then can we devise appropriate preventative strategies. There are two main methods for detection of medication harm; (1) retrospective review of medical records [30] (e.g., using coding data or a trigger tool as a starting point), or (2) prospective identification [31] (e.g., patient follow up by a trained clinician to identify medication harm close to the time it occurs). Whilst prospective methods are considered the gold standard [32] as they are likely to be more accurate, they are also more resource intensive. Retrospective detection poses a practical approach to harm detection [33]. However, all potential events should be evaluated with a comprehensive clinical record review and rated for causality (to identify a true link between the event and a medicine), severity, and preventability. Ideally, these processes should be done by trained clinicians, with multidisciplinary involvement (e.g., pharmacists, nurses, and physicians).
2.7 Assessment of Risk of Bias
The Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies (QATOCCS) [34] was used to critically appraise and evaluate the risk of bias for each study using the 14 key criteria. When conducting bias assessments, not all criteria were considered to be of equal importance, and a demerit point was awarded for every ‘No’, ‘Cannot Determine’ (CD), or ‘Not Reported’ (NR) response. For a study to receive an overall grade of ‘Good’ in the bias assessment, it should have two or fewer demerits; studies graded as ‘Fair’ had between three and six demerits; and if more than six demerits, studies were graded as ‘Poor’. Criteria listed as ‘Not Applicable’ (NA), did not carry a demerit. This overall grading method was devised through consensus by the research team.
3 Results
3.1 Study Selection
The literature search identified 3450 studies and after removing duplicates, 2685 remained. When screening titles and abstracts, 2661 were excluded. Of the 24 studies that remained, full-text articles were assessed for eligibility, and 15 were excluded. The remaining nine articles investigated medication harm in frail patient cohorts (using clearly defined criteria or validated measurements of frailty) and were included in the final review (Fig. 1)
An updated literature search (1 December 2021) undertaken has identified 206 (PubMed), 63 (CINAHL), 55 (Cochrane), and 235 (Embase) additional results. Of the identified 559 studies, duplicates were removed, and 408 remained. Four studies went through a further full-text review for eligibility, and none were included. The main reason for exclusion was that medication harm was not the primary/secondary outcome.
3.1.1 Study Design
The characteristics of the nine studies are shown in Table 1. Three studies used a retrospective cohort design (Cheong et al. [35], Cullinan et al. [36], Guo et al. [37]), and the remaining six studies (Athuraliya et al. [38], Stevenson et al. [39], Ruiz et al. [40], Schmader et al. [41] Hanlon et al. [42], Ekerstad et al. [43]) used a prospective design.
3.1.2 Study Characteristics
Four studies were conducted in the United States [37, 40,41,42], one in Singapore [35], one in Ireland [36], one in Australia [38], one in the United Kingdom (UK) [39], and one in Sweden [43]. Studies were conducted across different healthcare settings. Two studies included both inpatient and outpatient cohorts [37, 41], one study comprised only men in the community setting [38], three studies comprised only outpatient clinic attendees [35, 40, 42], and three only inpatients [36, 39, 43]. The mean age of study participants ranged from 65.5 to 89.7 years.
3.1.3 Study Gender
Three studies [35, 37, 43] reported having a greater proportion of female participants whilst four studies [39,40,41,42] reported more males in their study cohort. The study by Athuraliya et al. [38] aimed to examine the association of frailty with medication-related hospitalisation in community-dwelling older men, and therefore 100% of enrolled participants identified as males. One study [36] did not report any gender data.
Two studies [41, 42] used the same population with Schmader et al. [41] reporting no significant difference between genders (p = 0.78). The study by Ruiz et al. [40] specifically recruited individuals receiving palliative therapy for non–small-cell lung cancer (NSCLC). This may explain why they recruited more males, with men having a higher rate of tobacco smoking, a key risk factor for NSCLC [40].
3.1.4 Study Sample Size
All included studies reported sample sizes, which ranged from 50 to 37,799 participants. As six studies [35,36,37,38,39,40] comprised both frail and non-frail populations, sample size calculations would have been desirable in determining if the study had the required power to identify the pre-specified differences between the groups if they existed. However, only one study [35] reported a sample size calculation. Four studies [35, 36, 40, 43] indicated that the results of their findings may have been negatively impacted due to the small sample size and underpowered studies.
3.1.5 Study Setting
Four studies [35, 36, 39, 43] were conducted in the hospital inpatient setting, and two [40, 42] in hospital outpatient settings. The study by Ruiz et al. [40] and Hanlon et al. [42] were conducted in oncology outpatient clinics and veteran affairs medical centres, respectively. Two studies [37, 41] were conducted in both hospital inpatient and outpatient settings. Only one study [38] was conducted in a community setting where participants were from a population-based longitudinal study (Health In Men Study) identified from an electronic copy of the electoral roll [44].
3.1.6 Definition and Measurement of Frailty
The Clinical Frailty score developed by Rockwood et al. [23] presents the degree of frailty on a scale ranging from 1 to 9 (very fit to terminally ill). The FRAIL scale [45] consists of five domains: fatigue, resistance, ambulation, illness, and loss of weight. Each domain is given a score of 1 if it is present and individuals scoring ≥ 3 points are considered to be frail. The Frail Elderly Support Research (FRESH) group screening instrument [46] is an example that was developed based on the Fried phenotype model [22] with the addition of visual and cognitive impairment in the assessment. Individuals are determined to be frail if they meet ≥ 3 of the frailty indicators.
An example of the cumulative deficit model is the Fried frailty phenotype which considers the use of 21 variables to calculate predicted frailty probability with a cut-off score [47] and this score can be further specified to classify individuals with ≥ 0.20 as frail. Frailty measures could also be self-defined and not with modification or guidance of existing frailty tools. Some are assessed based on dependence in at least one activity of daily living [ADL], stroke within 3 months, previous falls, difficulty ambulating, malnutrition, dementia, depression, unplanned admission in the last 3 months, prolonged bed rest, or incontinence [41, 42]. Frailty is determined by meeting two out of the ten criteria.
The nine included studies utilised different methods, tools, and definitions in assessing frailty in the population. Six studies [35, 37,38,39,40, 43] reported using a validated frailty assessment to identify frailty. Cheong et al. [35] used the CFS, which is a validated measure by Rockwood et al. [23], which was able to predict the length of hospitalisation, functional decline, and 30-day outcome (readmission and mortality) after discharge with good reliability [48, 49]. Cullinan et al. [36] constructed a frailty index based on the Rockwood frailty index. However this was not validated [50]. Guo et al. [37] have used a validated algorithm that was developed based on the Fried frailty phenotype. The phenotype uses latent class analysis to internally validate theoretical construct, demonstrating high internal construct validity [51], and predictive validity was assessed [47]. Athuraliya et al. [38] used the FRAIL scale and this was internally validated in the same cohort of the Health In Men Study (HIMS) based on its predictive validity instead of construct validity [52].
Stevenson et al. [39] used a frailty index, which was internally validated using Kaplan–Meier analysis to evaluate the relationship between frailty and mortality [50]. Ruiz et al. [40] measured frailty phenotype using the Fried frailty index, which was validated and supported by other studies [22, 51, 53] on its concurrent and predictive validity. Two studies [41, 42] used non-validated self-defined criteria where they classified individuals as frail if they met two or more of the following ten criteria: dependence of at least one activity of daily living, stroke within 3 months prior to hospitalisation, previous falls, difficulty ambulating, malnutrition, dementia, depression, unplanned admission in the last 3 months, prolonged bed rest, or incontinence. Ekerstad et al. [43] used the Frail Elderly Support Research group screening instrument in evaluating frailty, supported by a study on its construct validity [46].
3.1.7 Definition and Measurement of Medication Harm Events
In this review, six studies (Guo et al. [37], Stevenson et al. [39], Ruiz et al. [40], Schmader et al. [41], Hanlon et al. [42], and Ekerstad et al. [43]) provided a clear definition of what constituted medication harm. Table 2 describes the key term used by each study for their measure of medication harm, as well as how harm was defined and measured. Nine studies [35,36,37,38,39,40,41,42,43] included ADRs as part of their outcome definition, two of which included harm from non-adherence to medications [39, 43]. Additionally, Stevenson et al. [39] included medication errors that resulted in actual medication harm.
Of the remaining four studies, three [35, 36, 42] did not provide any definition for the outcome of medication harm/ADR. Cheong et al. [35] provided no definition for ADRs or indeed how these ADRs were detected but did describe conducting a causality analysis using the Naranjo algorithm, and severity using the Hartwig criteria. Cullinan et al. [36] clearly detailed the identification of potentially inappropriate prescribing using the STOPP/START criteria (Screening Tool of Older Persons' Prescriptions/Screening Tool to Alert to Right Treatment) but failed to describe how ADRs were identified.
Two studies [41, 42] were based on the same study population. Schmader et al. [41] focused on the impacts of ADR by comparing events in groups of patients receiving geriatric care and usual inpatient care. Hanlon et al. [42] assessed the causality and preventability of ADR in the study population after a hospital stay. Hanlon et al. [42] did not report a definition of what constituted an ADR but did describe causality analysis based on assessments by blinded pairs of geriatrician and geriatric pharmacist.
The study by Athuraliya et al. [38] did not provide a clear definition of the primary outcome of ADEs but reported a wide range of International Classification of Disease version-10 (ICD-10) coded adverse events (both medication related and potentially medication related, e.g., renal failure). ICD-10 Y codes are synonymous with harm as a result of medication use and are identified and assigned to hospitalisations by clinical coding teams, generally for hospital financial reimbursement. Whilst a good crude measure of medication harm, the reliability of coding can be variable, for example depending on the expertise of the clinical coders and the accuracy of documentation in patient notes. A limitation of this study was the lack of clinical verification (i.e., review of patient records retrospectively) of the ICD-10 codes to confirm an actual medication harm event had taken place. Therefore, Athuraliya et al. [38] used the term ‘potential’ medication harm as they did not perform a subsequent causality analysis to confirm the coding data. A retrospective review of clinical coding data is a pragmatic method for detecting medication harm in large data sets but must be coupled with a robust clinical record review to confirm causality [54].
Guo et al. [37] defined medication harm to be bleeding and recurrent venous thromboembolism (VTE), also using a standard list of ICD codes to identify major bleeding (ICD-9) and recurrent VTE (ICD-10). They reported similar limitations, including that identification of medication harm using ICD codes may differ from the clinical diagnostic tools used in clinical trials. The study by Ruiz et al. [40] used toxicity as the measure of medication harm and applied a modified geriatric assessment risk score using cut offs for variables such as age (≥ 72 years), haemoglobin (b11 g/dL for men, b10 g/dL for women), creatinine clearance (< 34 mL/min per Jeliffe calculation), hearing impairment (fair or worse), number of falls in last 6 months (≥ 1), assistance with medications, limitation in walking one block, and decreased social activity because of physical/emotional health to determine the grade of toxicity from chemotherapy.
3.2 Causality Analysis
Five studies [35, 39, 41,42,43] rated causality between medication use and harm using tools comprising the Naranjo algorithm [55], Hallas criteria [56], and physician–pharmacist pairs using clinical judgement [57]. In the only study that used more than one tool concurrently, Ekerstad et al. [43] found rates of causality for the Naranjo scale [55], clinical judgement, and Hallas criteria [56] differed significantly: 9.4%, 13.5%, and 2.1%, respectively.
3.2.1 Severity
Only three studies [35, 39, 41] assessed the severity of ADRs, with Cheong et al. [35] employing the Hartwig and Siegel criteria [58] according to the clinical outcomes. Stevenson et al. [39] graded the severity of medication harm using the approach of Morimoto et al. [59]. Lastly, Schmader et al. [41] used the clinical judgement of a trained clinical pharmacist based on chart review or patient interview. The severity assessment is important to guide clinicians to carry out interventions based on the level of severity (e.g., minor, moderate, severe) [60].
3.2.2 Preventability
Three studies [39, 42, 43] reported preventability of medication harm, one based on consensus clinical judgement of an expert panel of physicians and pharmacists [42], and the others using the Hallas criteria with research pharmacists for cross-site case discussions [39] or with two independent clinicians to determine the preventability of hospital admissions [43].
3.2.3 Study Outcomes
Study outcomes relating to medication harm included medication-related hospitalisation, appropriateness of medications, chemotherapy-related toxicity, the incidence of major bleeding, clinically relevant non-major bleeding (CRNM), recurrent venous thromboembolism, and mortality (Table 3). Medication harm (e.g., ADE, ADR, other synonymous terms) was the primary outcome in eight studies [35, 37,38,39,40,41,42,43] and the secondary outcome in two studies [36, 37].
3.2.4 Frail and Non-Frail Populations
Six studies [35,36,37,38,39,40] included two groups of participants, one with frail and one without frail participants (or a so-called robust group). Cheong et al. [35] reported the prevalence of frailty as 83.3%, of whom 70% experienced more than one ADR, with more than 40% of the ADRs being mild to moderate in severity. The outcome of ADR experienced in each body system, namely cardiovascular, central nervous, endocrine, gastrointestinal, haematology, and renal was compared between the frail and robust group. Cheong et al. [35] found a higher incidence of medication harm related to cardiovascular medications in robust individuals compared to frail individuals (48% vs 24%, p = 0.03). However, the reverse was true for central nervous system medications where adverse events were more common in the frail (18.4%) than in robust individuals (18.4% vs 0%, p = 0.02).
Cullinan et al. [36] reported that patients who were deemed frail were twice as likely to develop ADRs (OR 2.6, CI 1.474–3.044, p < 0.0001) although the type of ADR was not explored. Even though the effect of experiencing ADRs was significant, the study failed to adjust for confounders such as polypharmacy. For a secondary outcome, Cullinan et al. [36] reported a significant correlation between FI score and potentially inappropriate prescribing (PIP), with frail individuals twice as likely to receive PIP (OR 2.1, CI 2.0–3.6, p < 0.0001).
Guo et al. [37] reported a large cohort size (N = 37799), comparing patients receiving apixaban vs warfarin. Among patients taking apixaban, frailty was associated with a 15% (HR 0.85, 95% CI 0.75–0.97) lower risk of CRNM bleeding, while non-frailty was associated with 32% lower risk (HR 0.68, 95% CI 0.59–0.78), as compared with patients taking warfarin. Apixaban was also associated with a lower risk of major bleeding and clinically CRNM, and a similar risk of recurrent venous thromboembolism as compared with warfarin (p values not reported), although there were no distinct comparisons between the frail and robust groups. Guo et al. [37] conducted 1:1 propensity score matching without replacement to balance baseline demographic and clinical characteristics between patients who were on apixaban as compared to warfarin.
Athuraliya et al. [38], found that frail men were at a higher risk of experiencing an ADR (OR 3.15, 95% CI 2.49–3.97, p < 0.001), ADE related hospitalisation (OR 6.83, CI 4.91–9.51) and mortality at 24 months (OR 3.14, 95% CI 2.28–4.33) compared to non-frail individuals. They [38] reported a significant association between frailty and medication harm-related outcomes and results were adjusted for several confounders including age, smoking status, and comorbidity, but not for polypharmacy.
Stevenson et al. [39] reported a significant relationship between medication-related harm and frailty (OR 10.06, 95% CI 2.06–49.26, p = 0.004), after adjusting for age, gender and polypharmacy. They also reported that with increasing polypharmacy, healthcare utilisation rates due to medication harm increased from 20 to 40%.
Ruiz et al. [40], reported that 27% of their oncology cohort were frail (FFI criteria ≥ 3). Of the frail population, 77% experienced grade 3–5 toxicity, and 23% experienced a grade 0–2 toxicity during the first cycle of chemotherapy (OR 7.03, 95% CI 1.11–44.5). They also demonstrated that FFI is a significant predictor of grade 3 or higher toxicities in the model which was adjusted for gender and current smoking at baseline (OR 10.22, 95% CI 1.24–84.27) in all patients who completed the second cycle of chemotherapy.
3.2.5 Frail Only Population
Three studies [41,42,43] only recruited frail patients, precluding any direct comparison in outcomes according to frailty. Schmader et al. [41] reported that outpatient geriatric management reduced serious ADR as compared with usual care, and geriatric management in both inpatient and outpatient settings reduced suboptimal prescribing. They also reported specialised geriatric outpatient clinics reduced the incidence of serious ADRs by 35% compared to usual outpatient care (p < 0.05).
The prospective study by Hanlon et al. [42] reported that 33% of participants experienced at least 1 ADR and 16% of the participants experienced at least 1 preventable ADR. ADRs are more common in frail elderly especially after hospitalisation, and in those with polypharmacy and receiving warfarin. Frail participants taking warfarin were at a 50% increased risk of ADR compared to participants not on warfarin (adjusted HR 1.51, 95% CI 1.22–1.87, p < 0.001).
Ekerstad et al. [43] reported that independent risk predictors for early rehospitalisation in their cohort were heart failure (OR 1.8, 95% CI 1.1–3.1) and anaemia (OR 2.3, 95% CI 1.3–4.0).
3.3 Risk of Bias
Using the QATOCCS [34] to categorise studies as “good (0-2 demerits), fair (3-5 demerits), or poor (≥ 6 demerits)”, the average number of demerits across studies was 4 points, indicating fair quality (Table 4), with demerits relating to lack of reporting of methods or results.
Only two of the nine studies [41, 43] met the criteria for “good” suggesting a low risk of bias, with limitations from failure to: examine the effects in different levels of medication exposure (i.e., drug dosage: low/medium/high dose) [41, 43]; to assessing medication harm more than once during the course of the study period [41, 43]; and not reporting if assessors were blinded from ADR assessment, and non-adherence contributing to rehospitalisation, when applying assessment tools [43]. However, examining medication effects from different levels of medication exposure is often not feasible in medication harm studies. The major strengths of these two studies [41, 43] were clear research aims; adjustment for confounders in predicting ADR and early rehospitalisation risk respectively; and sufficient timeframe to determine the association between the exposure and outcome. Schmader et al. [41] measured hospitalisation as the study outcome and allocated 12 months follow up after randomisation to ensure the association between the different types of care (geriatric/usual) and hospitalisation were appropriately examined. As early rehospitalisation was the study outcome, the authors [43] defined medication-related rehospitalisation within 30 days of discharge, which allowed a sufficient timeframe to assess the association between frailty and early rehospitalisation.
Six studies [35, 37,38,39,40, 42] were rated as “fair”. Among the studies that were rated “fair”, had similar limitations as those rated as “good”. Additionally, other common limitations include the lack of sample size justification and consideration of timeframe to reasonably identify medication harm from medication exposure. One study [36] was rated as “poor” due to unclear specification of the study population according to explicit selection criteria, lack of documentation on what adverse outcomes the study team considered to be ADR, and failure to adjust for potential confounders (polypharmacy).
3.4 Medications and Associated Harm Events
The types of harm and classes of medicines involved are listed in Table 5. Five studies [35, 38, 41,42,43] reported CVS medications which included classes such as angiotensin-converting-enzyme inhibitors (ACEI), angiotensin II receptor blockers (ARB), beta-blockers, calcium channel blockers, and digoxin. Two studies involved specific medication classes: Guo et al. [37] studied anticoagulants (apixaban and warfarin) and Ruiz et al. [40] studied the adverse effects of chemotherapy. Cullinan et al. [36] and Stevenson et al. [39] did not report the type or incidence of medication harm.
Among the nine studies, five studies [35, 37, 38, 40, 41] quantified the medication harm by listing the drug and/or the drug class that contributed to the medication harm (e.g., non-steroidal anti-inflammatory drugs & ACEI causing acute kidney injury). However, only Cheong et al. [35] reported the specific rates of medication harm to individual organ systems (cardiovascular, central nervous system, endocrine, gastrointestinal, haematology, renal) between the individual frail and non-frail populations. The study by Athuraliya et al. [38] observed that benzodiazepines and antihistamines were more commonly used among frail men. The other three studies [37, 40, 41] did not report the specific medication/medication class associated with each medication harm that was listed. The remaining four studies [36, 39, 42, 43] did not quantify medication harm by listing medications that contribute to an ADR.
Cheong et al. [35], Guo et al. [37], and Ekerstad et al. [43] reported the correlation between harm and the suspected medication. Three studies [38, 41, 42] listed the medications and medication harms, but without reporting a statistical association. Ekerstad et al. [43] also reported that underuse of evidence-based drug treatment could have a similar impact to ADR in leading to patient rehospitalisation, which affected 19.8% of patients.
The most common type of medication harm was cardiovascular ADRs, reported in six studies [35, 37, 40,41,42,43], with the second most common event being bleeding, reported in four studies [35, 37, 41, 43], and kidney injury, reported in four studies [35, 38, 41, 42]. Guo et al. [37] and Ruiz et al. [40] investigated anticoagulant and chemotherapy treatment, respectively. Therefore, the medication involved in both studies may not be indicative of the medications that are generally used in the geriatric population.
4 Discussion
This scoping review identified nine studies evaluating medication harm in frail patients, of which five [35,36,37,38,39] showed frailty has a statistically significant association with medication harm. Six studies [35,36,37,38,39,40] included both frail and non-frail cohorts, and three studies were conducted in a frail-only population. Only six studies [37,38,39,40,41, 43] performed statistical adjustments for baseline characteristics (i.e., age, gender, smoking status, comorbidity) and were independently correlated with medication harm. As polypharmacy is more prevalent in frail individuals compared with non-frail individuals [61], not statistically adjusting for polypharmacy may lower the internal validity of the studies, which reduces the generalisability of findings. Without adjusting for confounders, studies could only provide a crude association of frailty with medication harm. However, of the nine studies, only one [39] incorporated polypharmacy in their logistic regression model and was able to report the significant association of frailty with medication harm independent of the number of medications. Polypharmacy is a known variable that could potentially cause medication harm, making it inconclusive to determine if the association between medication harm and frailty was due to the presence of polypharmacy. We stress the need to use a multivariate analysis to determine the relationship between each variable’s contribution to frailty/medication harm.
4.1 Study Design and Characteristics
All nine studies used an observational study design. To best evaluate frailty as an independent risk factor for medication harm, a prospective cohort study recruiting both frail and robust patients with a justified sample size would be optimal. It should use sound methods for the detection of medication harm, incorporate other influential variables (e.g., polypharmacy) and adjust for key confounders to determine if frailty is independently associated with medication harm.
In our review, the mean patient age ranged from 65.5 to 89.7 years old. To date, studies of frailty are predominantly in older cohorts, and older age has been associated with both increased frailty and medication harm [12, 20]. However, younger adults are also at risk of both frailty and medication harm [62], and hence excluding younger individuals may not accurately portray the association of frailty with medication harm [63]. In a review, Spiers et al. [64] found no frailty measures that were designed and validated to identify frailty exclusively in young individuals (18–59 years old). Most existing literature evaluating frailty includes older participants [65, 66], and little is known about the prevalence of frailty and adverse outcomes in younger populations.
4.2 Definitions of Frailty
A gold standard method for frailty measurement has not been determined [67]. In our review, seven different assessment methods/tools were used to measure frailty. These methods included the phenotypic models [37] frailty index (accumulation of health deficits) [36, 39, 40], CFS [35], FRAIL scale [38], Frail Elderly Support Research (FRESH) group screening instrument [43], and self-defined assessment [41, 42]. This makes the comparison of frailty challenging, in particular where self-defined tools were used [41, 42]. A recent review evaluating 35 frailty scores found that agreement between frailty measures varies widely (measured using Cohen’s Kappa), and that agreement was highest with an accumulation of deficit-type scores [68]. This heterogeneity makes comparing research across studies of frail populations difficult. Even though there is no standardised method for selecting a frailty tool [50], researchers should utilise a validated measure to ensure reliability and enhance the generalisability of their findings. Future work should seek to endorse an established, validated tool to measure and report frailty in clinical research that can be easily applied across care settings. This will also assist with quantifying frailty as part of routine clinical care (e.g., in hospitals), where it may be incorporated within a risk prediction model to prioritise high-risk patients for timely and targeted interventions by the multidisciplinary team [69]. With the increasing availability of real-time electronic patient data, risk prediction models are an emerging area of research that present a potential intervention to improving patient safety [70].
4.3 Evaluating Medication Harm
Studies in our scoping review show that frailty has a significant association with poor medication outcomes (i.e., hospitalisation, toxicity, bleeding), and an increased risk of medication harm in frail versus non-frail cohorts. However, in studies that enrolled only frail populations, a comparison cannot be made with non-frail individuals, and these studies are only suitable as a broad guide for the incidence of medication harm in frail individuals. A study [71] investigating the association between frailty measures in predicting mortality, nursing home transfer, and hospitalisation has reported similar limitations in study design, with a lack of non-frail participants limiting the generalisability of their findings.
Overall, studies reported a wide range of medication harm event rates, likely due to differences in methods of measurement, a lack of consensus in definitions for harm, and limited guidelines for data collection in this field. Similar disparities in event rates have been reported by other studies related to medication harm [14, 72]. We urge key safety bodies to lobby for the harmonisation of metrics in this area to facilitate robust research.
4.4 Medications and Associated Harm
To obtain a true sense of the incidence of medication harm, it is important to capture information on the frequency and types of medicines that were prescribed for the cohort. Whilst most studies [35, 37, 38, 40, 41] included information on types of medications that resulted in harm, there was no information on the frequency of prescribing. This limitation should be addressed in future research in this area.
4.5 Causality Analysis
Causality analysis is important in confirming a link between medications and potential patient harm [73]. In our scoping review, only five of nine studies [35, 39, 41,42,43] used a causality analysis tool to evaluate medication harm, and only one study [39] used the Naranjo algorithm coupled with clinical judgement by an independent panel (considered the gold standard) [74]. Studies that did not include causality analysis [36, 37] were limited in the accuracy of their outcome measurement, as an event may not be true medication harm. The three methods used to conduct a causality analysis included the Naranjo algorithm [55], Hallas criteria [56], and clinical judgement. Clinical judgement was used by one study [43] but when used alone this is a subjective method that can be confounded by assessor bias.
Whilst different causality assessment tools use similar criteria, their application and scoring systems differ (e.g., Naranjo is a score-based system with different cut-off points depending on ten criteria, whereas the Hallas has only five criteria applied using expert judgement). Limitations in causality analysis tools have been described by previous studies [73, 75]. Ideally, causality analysis should be undertaken using a validated tool coupled with clinical judgement [76]. Clinical judgement should be conducted with an experienced panel of clinicians that are multidisciplinary, and where inter-rater reliability is considered in evaluating assessor bias [77]. Studies should also establish standardised protocols, detailing the steps and assessors involved in rating events to ensure transparent reporting. In this review, only two studies [41, 42] reported using an expert panel coupled with a causality analysis tool.
4.6 Polypharmacy and Frailty
Polypharmacy is known to increase an individual’s risk of medication harm [78,79,80]. Similarly, frailty also appears to be associated with an increased risk of medication harm [39, 81]. Polypharmacy, frailty, and medication harm are intertwined constructs. Therefore, we need adequately sized and robust studies that have investigated medication harm and made the statistical adjustment to eliminate the potential confounding effects of polypharmacy. In this review, only Stevenson et al. [39] demonstrated an independent relationship with medication harm, with frail individuals being ten times more likely to experience medication harm. Further research is needed to confirm this association.
4.7 Future Research
Rigorous prospective studies that comprehensively evaluate medication harm in sufficiently powered studies with groups of frail and non-frail patients, using a broad range of medications likely to be representative of the population as a whole, are needed to determine if frailty is an independent predictor of medication-related harm. Studies could further examine and classify medications/medication classes most associated with ADRs, especially in the frail population. The identification of high-risk medications could serve as a useful tool to guide prescribing decisions.
5 Conclusion
Our scoping review identified and appraised nine studies that evaluated the relationship between frailty and medication harm. In the six studies comparing frail versus non-frail cohorts, frail individuals were reported to have a higher incidence of medication harm, harm-related hospitalisation, and mortality, and these risks were magnified if patients were receiving CNS-acting medications or warfarin. When evaluating the association between frailty and medication harm, only one study identified a relationship independent of polypharmacy.
With continued research in this field, frailty screening tools could be evaluated as part of pharmacist interventions to inform the risk of medication harm in individuals. However, studies need to be comprehensive in their evaluation to encompass the types of medicines involved, the definition of harm, and other key measures such as severity and preventability of medication harm.
Change history
16 June 2022
A Correction to this paper has been published: https://doi.org/10.1007/s40266-022-00956-9
References
Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55(5):780–91.
Vermeiren S, Vella-Azzopardi R, Beckwée D, Habbig AK, Scafoglieri A, Jansen B, et al. Frailty and the prediction of negative health outcomes: a meta-analysis. J Am Med Dir Assoc. 2016;17(12):1163 (e1–e17).
Thompson MQ, Theou O, Karnon J, Adams RJ, Visvanathan R. Frailty prevalence in Australia: findings from four pooled Australian cohort studies. Australas J Ageing. 2018;37(2):155–8.
Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):255–63.
Heppenstall CP, Wilkinson TJ, Hanger HC, Keeling S. Frailty: dominos or deliberation? N Z Med J. 2009;122(1299):42–53.
Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;27(1):17–26.
Xue Q-L. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27(1):1–15.
Rockwood K. Conceptual models of frailty: accumulation of deficits. Can J Cardiol. 2016;32(9):1046–50.
Archibald M, Lawless M, Ambagtsheer RC, Kitson A. Older adults’ understandings and perspectives on frailty in community and residential aged care: an interpretive description. BMJ Open. 2020;10(3): e035339.
Rohrmann S. Epidemiology of frailty in older people. Adv Exp Med Biol. 2020;1216:21–7.
Hubbard RE, O’Mahony MS, Woodhouse KW. Medication prescribing in frail older people. Eur J Clin Pharmacol. 2013;69(3):319–26.
Hughes R, Blegen M. Chapter 37: Medication administration safety. Patient safety and quality: an evidence-based handbook for nurses. 2008.
Falconer N, Barras M, Martin J, Cottrell N. Defining and classifying terminology for medication harm: a call for consensus. Eur J Clin Pharmacol. 2019;75(2):137–45.
Parekh N, Ali K, Page A, Roper T, Rajkumar C. Incidence of medication-related harm in older adults after hospital discharge: a systematic review. J Am Geriatr Soc. 2018;66(9):1812–22.
Luke S, Ane A, Nicolaas S. K. The economics of patient safety: Strengthening a value-based approach to reducing patient harm at national level. OECD Health Working Papers, No 96. 2017.
Zazzara MB, Palmer K, Vetrano DL, Carfì A, Onder G. Adverse drug reactions in older adults: a narrative review of the literature. Eur Geriatr Med. 2021;12(3):463–73.
Hodkinson A, Tyler N, Ashcroft DM, Keers RN, Khan K, Phipps D, et al. Preventable medication harm across health care settings: a systematic review and meta-analysis. BMC Med. 2020;18(1):313.
World Health Organization. Medication without harm [cited 22 Aug 2021]. https://www.who.int/initiatives/medication-without-harm.
Parekh N, Ali K, Stevenson JM, Davies JG, Schiff R, Van der Cammen T, et al. Incidence and cost of medication harm in older adults following hospital discharge: a multicentre prospective study in the UK. Br J Clin Pharmacol. 2018;84(8):1789–97.
Chen Y, Zhu LL, Zhou Q. Effects of drug pharmacokinetic/pharmacodynamic properties, characteristics of medication use, and relevant pharmacological interventions on fall risk in elderly patients. Ther Clin Risk Manag. 2014;10:437–48.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56.
Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–95.
Kulminski AM, Ukraintseva SV, Kulminskaya IV, Arbeev KG, Land K, Yashin AI. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the Cardiovascular Health Study. J Am Geriatr Soc. 2008;56(5):898–903.
World Health Organization. International drug monitoring: the role of national centres, report of a WHO meeting [held in Geneva from 20 to 25 September 1971]. Geneva: World Health Organization; 1972.
Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000;356(9237):1255–9.
Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13(3):176–81.
Hakkarainen KM, Hedna K, Petzold M, Hägg S. Percentage of patients with preventable adverse drug reactions and preventability of adverse drug reactions—a meta-analysis. PLoS One. 2012;7(3): e33236.
Coleman JJ, Pontefract SK. Adverse drug reactions. Clin Med (Lond). 2016;16(5):481–5.
Howard I, Howland I, Castle N, Al Shaikh L, Owen R. Retrospective identification of medication related adverse events in the emergency medical services through the analysis of a patient safety register. Sci Rep. 2022;12(1):2622.
Falconer N, Spinewine A, Doogue MP, Barras M. Identifying medication harm in hospitalised patients: a bimodal, targeted approach. Ther Adv Drug Saf. 2020;11:2042098620975516.
Thiese MS. Observational and interventional study design types; an overview. Biochem Med (Zagreb). 2014;24(2):199–210.
Euser AM, Zoccali C, Jager KJ, Dekker FW. Cohort studies: prospective versus retrospective. Nephron Clin Pract. 2009;113(3):c214–7.
National Heart Lung and Blood Institute. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies [cited 04 Aug 2021]. http://www.nhlbi.nih.gov/health-pro/guidelines/indevelop/cardiovascular-risk-reduction/tools/cohort.
Cheong S, Goh W, Ong E, Abdul-Karim S, See L, Chong E. The prevalence of frailty and its association with adverse drug reactions in hospitalized older adults. J Geriatr Med Gerontol. 2019;5(3):1–8.
Cullinan S, O’Mahony D, O’Sullivan D, Byrne S. Use of a frailty index to identify potentially inappropriate prescribing and adverse drug reaction risks in older patients. Age Ageing. 2016;45(1):115–20.
Guo JD, Hlavacek P, Rosenblatt L, Keshishian A, Russ C, Mardekian J, et al. Safety and effectiveness of apixaban compared with warfarin among clinically-relevant subgroups of venous thromboembolism patients in the United States Medicare population. Thromb Res. 2021;198:163–70.
Athuraliya N, Etherton-Beer C. Health in Men Study: is frailty a predictor of medication-related hospitalization? QJM. 2022;115(2):84–90.
Stevenson JM, Parekh N, Chua K-C, Davies JG, Schiff R, Rajkumar C, et al. Frailty is a predictor of medication-related harm requiring healthcare utilisation: a multicentre prospective cohort study. medRxiv. 2021:2021.05.17.21257344.
Ruiz J, Miller AA, Tooze JA, Crane S, Petty WJ, Gajra A, et al. Frailty assessment predicts toxicity during first cycle chemotherapy for advanced lung cancer regardless of chronologic age. J Geriatr Oncol. 2019;10(1):48–54.
Schmader KE, Hanlon JT, Pieper CF, Sloane R, Ruby CM, Twersky J, et al. Effects of geriatric evaluation and management on adverse drug reactions and suboptimal prescribing in the frail elderly. Am J Med. 2004;116(6):394–401.
Hanlon JT, Pieper CF, Hajjar ER, Sloane RJ, Lindblad CI, Ruby CM, et al. Incidence and predictors of all and preventable adverse drug reactions in frail elderly persons after hospital stay. J Gerontol A Biol Sci Med Sci. 2006;61(5):511–5.
Ekerstad N, Bylin K, Karlson BW. Early rehospitalizations of frail elderly patients—the role of medications: a clinical, prospective, observational trial. Drug Healthc Patient Saf. 2017;9:77–88.
Norman PE, Flicker L, Almeida OP, Hankey GJ, Hyde Z, Jamrozik K. Cohort profile: The Health In Men Study (HIMS). Int J Epidemiol. 2009;38(1):48–52.
Aprahamian I, Cezar NODC, Izbicki R, Lin SM, Paulo DLV, Fattori A, et al. Screening for frailty with the frail scale: a comparison with the phenotype criteria. J Am Med Dir Assoc. 2017;18(7):592–6.
Kajsa E, Katarina W, Sten L, Synneve I-D. Screening for frailty among older emergency department visitors: validation of the new FRESH-screening instrument. BMC Emerg Med. 2016;16(1):27.
Segal JB, Chang HY, Du Y, Walston JD, Carlson MC, Varadhan R. Development of a claims-based frailty indicator anchored to a well-established frailty phenotype. Med Care. 2017;55(7):716–22.
Chong E, Ho E, Baldevarona-Llego J, Chan M, Wu L, Tay L. Frailty and risk of adverse outcomes in hospitalized older adults: a comparison of different frailty measures. J Am Med Dir Assoc. 2017;18(7):638.
Kahlon S, Pederson J, Majumdar SR, Belga S, Lau D, Fradette M, et al. Association between frailty and 30-day outcomes after discharge from hospital. CMAJ. 2015;187(11):799–804.
Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8(1):24.
Bandeen-Roche K, Xue Q-L, Ferrucci L, Walston J, Guralnik JM, Chaves P, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontolo Ser A. 2006;61(3):262–6.
Hyde Z, Flicker L, Almeida OP, Hankey GJ, McCaul KA, Chubb SAP, et al. Low free testosterone predicts frailty in older men: the health in men study. J Clin Endocrinol Metab. 2010;95(7):3165–72.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol Ser A. 2001;56(3):M146–57.
Falconer N, Spinewine A, Doogue MP, Barras M. Identifying medication harm in hospitalised patients: a bimodal, targeted approach. Ther Adv Drug Saf. 2020;11:2042098620975516.
Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–45.
Hallas J, Harvald B, Gram LF, Grodum E, Brøsen K, Haghfelt T, et al. Drug related hospital admissions: the role of definitions and intensity of data collection, and the possibility of prevention. J Intern Med. 1990;228(2):83–90.
Doherty MJ. Algorithms for assessing the probability of an adverse drug reaction. Respir Med CME. 2009;2(2):63–7.
Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992;49(9):2229–32.
Morimoto T, Gandhi TK, Seger AC, Hsieh TC, Bates DW. Adverse drug events and medication errors: detection and classification methods. Qual Saf Health Care. 2004;13(4):306–14.
Petrova G, Stoimenova A, Dimitrova M, Kamusheva M, Petrova D, Georgiev O. Assessment of the expectancy, seriousness and severity of adverse drug reactions reported for chronic obstructive pulmonary disease therapy. SAGE Open Med. 2017;5:2050312117690404.
Herr M, Robine JM, Pinot J, Arvieu JJ, Ankri J. Polypharmacy and frailty: prevalence, relationship, and impact on mortality in a French sample of 2350 old people. Pharmacoepidemiol Drug Saf. 2015;24(6):637–46.
Smart R, Carter B, McGovern J, Luckman S, Connelly A, Hewitt J, et al. Frailty exists in younger adults admitted as surgical emergency leading to adverse outcomes. J Frailty Aging. 2017;6(4):219–23.
Loecker C, Schmaderer M, Zimmerman L. Frailty in young and middle-aged adults: an integrative review. J Frailty Aging. 2021;10(4):327–33.
Spiers GF, Kunonga TP, Hall A, Beyer F, Boulton E, Parker S, et al. Measuring frailty in younger populations: a rapid review of evidence. BMJ Open. 2021;11(3): e047051.
Thillainadesan J, Scott IA, Le Couteur DG. Frailty, a multisystem ageing syndrome. Age Ageing. 2020;49(5):758–63.
Nicholson C, Gordon AL, Tinker A. Changing the way “we” view and talk about frailty…. Age Ageing. 2017;46(3):349–51.
Lee H, Lee E, Jang IY. Frailty and comprehensive geriatric assessment. J Korean Med Sci. 2020;35(3): e16.
Aguayo GA, Donneau AF, Vaillant MT, Schritz A, Franco OH, Stranges S, et al. Agreement between 35 published frailty scores in the general population. Am J Epidemiol. 2017;186(4):420–34.
Falconer N, Barras M, Abdel-Hafez A, Radburn S, Cottrell N. Development and validation of the Adverse Inpatient Medication Event model (AIME). Br J Clin Pharmacol. 2021;87(3):1512–24.
Falconer N, Barras M, Cottrell N. Systematic review of predictive risk models for adverse drug events in hospitalized patients. Br J Clin Pharmacol. 2018;84(5):846–64.
Rosenberg T, Montgomery P, Hay V, Lattimer R. Using frailty and quality of life measures in clinical care of the elderly in Canada to predict death, nursing home transfer and hospitalisation - the frailty and ageing cohort study. BMJ Open. 2019;9(11): e032712.
Dean B. Adverse drug events: what’s the truth? Qual Saf Health Care. 2003;12(3):165.
Belhekar MN, Taur SR, Munshi RP. A study of agreement between the Naranjo algorithm and WHO-UMC criteria for causality assessment of adverse drug reactions. Indian J Pharmacol. 2014;46(1):117–20.
Hey-Hadavi J, Seekins D, Palmer M, Coffey D, Caminis J, Abdullaev S, et al. Overview of causality assessment for drug-induced liver injury (DILI) in clinical trials. Drug Saf. 2021;44(6):619–34.
Mittal N, Gupta MC. Comparison of agreement and rational uses of the WHO and Naranjo adverse event causality assessment tools. J Pharmacol Pharmacother. 2015;6(2):91–3.
Khan LM, Al-Harthi SE, Osman A-MM, Sattar MAAA, Ali AS. Dilemmas of the causality assessment tools in the diagnosis of adverse drug reactions. Saudi Pharm J. 2016;24(4):485–93.
Belur J, Tompson L, Thornton A, Simon M. Interrater reliability in systematic review methodology: exploring variation in coder decision-making. Sociol Methods Res. 2018;50(2):837–65.
Halli-Tierney A, Scarbrough C, Carroll DG. Polypharmacy: evaluating risks and deprescribing. Am Fam Physician. 2019;100(1):32–8.
Valenza PL, McGinley TC, Feldman J, Patel P, Cornejo K, Liang N, et al. Dangers of polypharmacy. Vignettes in Patient Safety-Volume 1: IntechOpen; 2017.
Davies EA, O’Mahony MS. Adverse drug reactions in special populations—the elderly. Br J Clin Pharmacol. 2015;80(4):796–807.
Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394(10206):1365–75.
Strand LM, Morley PC, Cipolle RJ, Ramsey R, Lamsam GD. Drug-related problems: their structure and function. Dicp. 1990;24(11):1093–7.
Acknowledgements
The authors would like to thank the Princess Alexandra Research Foundation for their generous support that allowed the time to conduct research in the field of patient safety. We are also sincerely thankful to Mrs Christine Dalais, our liaison librarian at The University of Queensland, who provided guidance and assisted with the literature search.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics declaration
Not applicable.
Funding
By Princess Alexandra Research Foundation.
Authorship and conflict of interest
The authors have no conflicts of interest to declare.
Ethics approval
Not applicable.
Consent to participate and consent to publish
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
JL, MB, DL, NF designed and conducted the study. Data collection and analysis were undertaken by JL, MB, DL, NF, LH. The first draft of the manuscript was written by JL, MB, NF, and all authors commented on previous versions of the manuscript. All authors revised the article and approved the final manuscript.
Additional information
The original Online version of this article was revised: The Online supplementary file has been published with incorrect reference in the Appendix 2.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Lam, J.Y.J., Barras, M., Scott, I.A. et al. Scoping Review of Studies Evaluating Frailty and Its Association with Medication Harm. Drugs Aging 39, 333–353 (2022). https://doi.org/10.1007/s40266-022-00940-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-022-00940-3