Abstract
Background
The impact of aging on the effectiveness of immune checkpoint inhibitors (ICIs) remains controversial, and little is known on the subject in adults aged ≥ 75 years.
Objective
The objective of this comprehensive meta-analysis was to assess the efficacy of ICIs in patients aged ≥ 75 years.
Methods
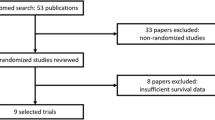
We performed a meta-analysis of published randomized controlled trials concerning ICIs (as monotherapy or in combination) versus standard therapy in patients with advanced solid tumors between January 2010 and January 2020. We compared overall survival between older (aged ≥ 75 years) and younger (< 75 years) patients. Hazard ratios (HRs) and 95% confidence intervals (CIs) were collected and pooled. The secondary endpoint focused on the impact of the use of ICIs in first- and second-line settings.
Results
In total, 15 phase III studies evaluating anti-programmed cell death 1 (anti-PD-1) (nivolumab or pembrolizumab), anti-programmed cell death ligand 1 (anti-PD-L1) (atezolizumab or avelumab), or anti-cytotoxic T lymphocyte antigen 4 (anti-CTLA-4) (ipilimumab) therapies were included. Enrolled patients had non-small-cell lung cancer, renal cell carcinoma, melanoma, head and neck squamous cell carcinoma, or gastric cancer. Eight studies assessed treatment in the first-line setting and seven in the second-line setting. The median age was 64 years, with 906 patients aged ≥ 75 years (552 in first line, 354 in second line) and 8741 were aged < 75 years (4992 in first line, 3749 in second line). In the first-line setting, HRs for death were 0.78 (95% CI 0.61–0.99) in patients aged ≥ 75 years versus 0.84 (95% CI 0.71–1.00) in those aged < 75 years. In the second-line setting, HRs for death were 1.02 (95% CI 0.77–1.36) in patients aged ≥ 75 years versus 0.68 (95% CI 0.61–0.75) in those aged < 75 years, with a statistically significant difference observed between subgroups (p = 0.009 for interaction).
Conclusions
ICIs appear to be effective in patients aged ≥ 75 years. However, the survival benefit is mainly observed in first-line treatment and remains unclear in the second-line setting.




Similar content being viewed by others
References
Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–92.
Escudier B. Combination therapy as first-line treatment in metastatic renal-cell carcinoma. N Engl J Med. 2019;380(12):1176–8.
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23.
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–20.
Soto-Perez-de-Celis E, Li D, Yuan Y, Lau YM, Hurria A. Functional versus chronological age: geriatric assessments to guide decision making in older patients with cancer. Lancet Oncol. 2018;19(6):e305–e316316.
Pawelec G. Does patient age influence anti-cancer immunity? Semin Immunopathol. 2019;41:125–31.
Nishijima TF, Muss HB, Shachar SS, Moschos SJ. Comparison of efficacy of immune checkpoint inhibitors (ICIs) between younger and older patients: a systematic review and meta-analysis. Cancer Treat Rev. 2016;45:30–7.
Elias R, Giobbie-Hurder A, McCleary NJ, Ott P, Hodi FS, Rahma O. Efficacy of PD-1 & PD-L1 inhibitors in older adults: a meta-analysis. J Immunother Cancer. 2018;6(1):26.
Ninomiya K, Oze I, Kato Y, Kubo T, Ichihara E, Rai K, et al. Influence of age on the efficacy of immune checkpoint inhibitors in advanced cancers: a systematic review and meta-analysis. Acta Oncol. 2020;59(3):249–56.
Yee KWL, Pater JL, Pho L, Zee B, Siu LL. Enrollment of older patients in cancer treatment trials in Canada: why is age a barrier? J Clin Oncol. 2003;21(8):1618–23.
Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004;22(22):4626–31.
Ferris RL, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–67.
Kang Y-K, Boku N, Satoh T, Ryu M-H, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461–71.
Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–35.
Reck M, Luft A, Szczesna A, Havel L, Kim S-W, Akerley W, et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol. 2016;34(31):3740–8.
Govindan R, Szczesna A, Ahn M-J, Schneider C-P, Gonzalez Mella PF, Barlesi F, et al. Phase III trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non-small-cell lung cancer. J Clin Oncol. 2017;35(30):3449–577.
Barlesi F, Vansteenkiste J, Spigel D, Ishii H, Garassino M, de Marinis F, et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol. 2018;19(11):1468–79.
Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim S-W, Carcereny Costa E, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381:2020–31.
Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, Han J-Y, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–50.
Mok TSK, Wu Y-L, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–30.
Spigel D, de Marinis F, Giaccone G, Reinmuth N, Vergnenegre A, Barrios CH, et al. LBA78IMpower110: Interim overall survival (OS) analysis of a phase III study of atezolizumab (atezo) vs platinum-based chemotherapy (chemo) as first-line (1L) treatment (tx) in PD-L1–selected NSCLC. Ann Oncol [Internet]. 2019;30(Supplement_5). Available from: https://academic.oup.com/annonc/article/doi/10.1093/annonc/mdz293/5577589
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–39.
Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–33.
Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–13.
Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–90.
Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–30.
Bang Y-J, Ruiz EY, Van Cutsem E, Lee K-W, Wyrwicz L, Schenker M, et al. Phase 3, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment for patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol. 2018;29(10):2052–60.
Cohen EEW, Soulières D, Le Tourneau C, Dinis J, Licitra L, Ahn M-J, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393(10167):156–67.
Long GV, Atkinson V, Ascierto PA, Robert C, Hassel JC, Rutkowski P, et al. Effect of nivolumab on health-related quality of life in patients with treatment-naïve advanced melanoma: results from the phase III CheckMate 066 study. Ann Oncol. 2016;27(10):1940–6.
Cella D, Grünwald V, Nathan P, Doan J, Dastani H, Taylor F, et al. Quality of life in patients with advanced renal cell carcinoma given nivolumab versus everolimus in CheckMate 025: a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17(7):994–1003.
Reck M, Taylor F, Penrod JR, DeRosa M, Morrissey L, Dastani H, et al. Impact of nivolumab versus docetaxel on health-related quality of life and symptoms in patients with advanced squamous non-small cell lung cancer: results from the CheckMate 017 study. J Thorac Oncol. 2018;13(2):194–204.
Li J, Gu J. Efficacy of immune checkpoint inhibitors in cancer patients of different ages: a meta-analysis. Future Oncol. 2019;15(31):3633–46.
Fane M, Weeraratna AT. How the ageing microenvironment influences tumour progression. Nat Rev Cancer. 2020;20(2):89–106.
Bastholt L, Schmidt H, Bjerregaard JK, Herrstedt J, Svane IM. Age favoured overall survival in a large population-based Danish patient cohort treated with anti-PD1 immune checkpoint inhibitor for metastatic melanoma. Eur J Cancer. 1990;2019(119):122–31.
Ibrahim T, Mateus C, Baz M, Robert C. Older melanoma patients aged 75 and above retain responsiveness to anti-PD1 therapy: results of a retrospective single-institution cohort study. Cancer Immunol Immunother. 2018;67(10):1571–8.
Nosaki K, Saka H, Hosomi Y, Baas P, de Castro G, Reck M, et al. Safety and efficacy of pembrolizumab monotherapy in elderly patients with PD-L1-positive advanced non-small-cell lung cancer: Pooled analysis from the KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042 studies. Lung Cancer. 2019;135:188–95.
Author information
Authors and Affiliations
Contributions
TL, SC, and GDG designed the research, collected the data, analyzed and interpreted the data, and wrote the manuscript. SC and GDG contributed to the collection of data and revision of the manuscript. KC and VFD participated in the elaboration of the research design and revision of the manuscript.
Corresponding author
Ethics declarations
Funding
No sources of funding were used to conduct this study or prepare this manuscript.
Conflict of interest
TL, GDG, KC, VFD, and SC have no conflicts of interest that might be relevant to the contents of this article.
Rights and permissions
About this article
Cite this article
Landre, T., Des Guetz, G., Chouahnia, K. et al. Immune Checkpoint Inhibitors for Patients Aged ≥ 75 Years with Advanced Cancer in First- and Second-Line Settings: A Meta-Analysis. Drugs Aging 37, 747–754 (2020). https://doi.org/10.1007/s40266-020-00788-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-020-00788-5