Abstract
Background
Age-related changes in the concentration–effect relationship of (+)-O-desmethyl-tramadol [(+)-ODM], tramadol’s active metabolite, are not documented in the elderly.
Objective
The objective of this study was to characterize, in elderly and young subjects, the (+)-ODM pharmacokinetic and pharmacodynamic relationship to examine the effect of age after single-dose administration of tramadol 200 mg extended-release tablets.
Methods
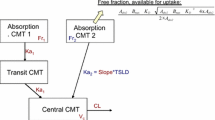
A population analysis of a double-blind, randomized, placebo-controlled, two-period cross-over study including 13 elderly (aged ≥75 years) subjects with mild renal insufficiency and 16 young (aged 18–40 years) subjects was conducted. For 48 h post-dose, blood samples were collected and pain tolerance thresholds measured using an electrically stimulated pain model. A pharmacokinetic/pharmacodynamic model incorporating a one-compartment pharmacokinetic model for (+)-ODM parameterized with first-order formation rate, clearance (CL/fm), volume of distribution (V/fm) and a sigmoid maximum effect (Emax) model incorporating baseline (E0) and placebo effect was used.
Results
Maximum plasma concentrations of (+)-ODM occurred later and plasma concentrations declined more slowly in the elderly than in young subjects. In the elderly, V/fm was 76% larger and CL/fm 16% slower. Baseline (E0) and sensitivity (C50) for pain tolerance were similar between young and elderly subjects. However, the Emax parameter was 2.5 times higher in the elderly and maximum possible treatment-related effect was 169 (135–221) in the young and 194 (149–252) in the elderly; that is, 15% higher in the elderly.
Conclusions
This exploratory analysis suggests that age-related differences exist in the distribution and elimination of (+)-ODM, including a 76% larger distribution outside the central compartment and 16% slower clearance of (+)-ODM. These pharmacokinetic changes are associated with a 15% higher maximum possible treatment-related effect and carry the potential for greater efficacy but also the potential for increased side effects at the same dose in elderly subjects.
Clinicaltrials.gov identifier: NCT02329561.


Similar content being viewed by others
References
McLachlan AJ, Bath S, Naganathan V, Hilmer SN, Le Couteur DG, Gibson SJ, et al. Clinical pharmacology of analgesic medicines in older people: impact of frailty and cognitive impairment. Br J Clin Pharmacol. 2011;71(3):351–64.
Pergolizzi J, Boger RH, Budd K, Dahan A, Erdine S, Hans G, et al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone). Pain Pract. 2008;8(4):287–313.
Chung J. Geriatric clinical pharmacology and clinical trials in the elderly. Transl Clin Pharmacol. 2014;22(2):64–9.
Chien JY, Ho RJ. Drug delivery trends in clinical trials and translational medicine: evaluation of pharmacokinetic properties in special populations. J Pharm Sci. 2011;100(1):53–8.
Lee CR, McTavish D, Sorkin EM. Tramadol. A preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in acute and chronic pain states. Drugs. 1993;46(2):313–40.
Paeck T, Ferreira ML, Sun C, Lin CW, Tiedemann A, Maher CG. Are older adults missing from low back pain clinical trials? A systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2014;66(8):1220–6.
Cepeda MS, Camargo F, Zea C, Valencia L. Tramadol for osteoarthritis: a systematic review and metaanalysis. J Rheumatol. 2007;34(3):543–55.
Chaparro LE, Furlan AD, Deshpande A, Mailis-Gagnon A, Atlas S, Turk DC. Opioids compared with placebo or other treatments for chronic low back pain: an update of the Cochrane Review. Spine (Phila Pa 1976). 2014;39(7):556–63.
Duhmke RM, Cornblath DD, Hollingshead JR. Tramadol for neuropathic pain. Cochrane Database Syst Rev. 2004(2):CD003726.
Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an ‘atypical’ opioid analgesic. J Pharmacol Exp Ther. 1992;260(1):275–85.
Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL, et al. Complementary and synergistic antinociceptive interaction between the enantiomers of tramadol. J Pharmacol Exp Ther. 1993;267(1):331–40.
American Geriatrics Society Panel on the Pharmacological Management of Persistent Pain in Older P. Pharmacological management of persistent pain in older persons. Pain Med (Malden, Mass). 2009;10(6):1062–83.
Cossmann M, Kohnen C, Langford R, McCartney C. Tolerance and safety of tramadol use. Results of international studies and data from drug surveillance. Drugs. 1997;53(Suppl 2):50–62.
Bush D. The CBHSQ Report: Emergency Department Visits for Adverse Reactions Involving the Pain Medication Tramadol (2015). Rockville, MD.: Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. 2011.
Bianchi MFP, Panerai AE. The levels of tramadol and its M1 metabolite in the plasma, cerebrospinal fluid and midbrainf following acute tramadol administration in rats. Analgesia. 2002;6:39–42.
Koga A, Fujita T, Totoki T, Kumamoto E. Tramadol produces outward currents by activating mu-opioid receptors in adult rat substantia gelatinosa neurones. Br J Pharmacol. 2005;145(5):602–7.
Frink MC HH, Englberger W et al. Influence of tramadol on neurotransmitter systems of the rat brain. Arzneimittel-Forschung. 1996;46(11):1029–36.
Hennies HH, Friderichs E, Schneider J. Receptor binding, analgesic and antitussive potency of tramadol and other selected opioids. Arzneimittel-Forschung. 1988;38(7):877–80.
Halfpenny DM, Callado LF, Hopwood SE, Bamigbade TA, Langford RM, Stamford JA. Effects of tramadol stereoisomers on norepinephrine efflux and uptake in the rat locus coeruleus measured by real time voltammetry. Br J Anaesth. 1999;83(6):909–15.
Bamigbade TA, Davidson C, Langford RM, Stamford JA. Actions of tramadol, its enantiomers and principal metabolite, O-desmethyltramadol, on serotonin (5-HT) efflux and uptake in the rat dorsal raphe nucleus. Br J Anaesth. 1997;79(3):352–6.
Lintz W, Barth H, Osterloh G, Schmidt-Bothelt E. Bioavailability of enteral tramadol formulations. 1st communication: capsules. Arzneimittel-Forschung. 1986;36(8):1278–83.
Lintz W, Erlacin S, Frankus E, Uragg H. Biotransformation of tramadol in man and animal (author’s transl). Arzneimittel-Forschung. 1981;31(11):1932–43.
Paar WD, Poche S, Gerloff J, Dengler HJ. Polymorphic CYP2D6 mediates O-demethylation of the opioid analgesic tramadol. Eur J Clin Pharmacol. 1997;53(3–4):235–9.
Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43(13):879–923.
Likar R, Wittels M, Molnar M, Kager I, Ziervogel G, Sittl R. Pharmacokinetic and pharmacodynamic properties of tramadol IR and SR in elderly patients: a prospective, age-group-controlled study. Clin Ther. 2006;28(12):2022–39.
Desmeules JA, Piguet V, Collart L, Dayer P. Contribution of monoaminergic modulation to the analgesic effect of tramadol. Br J Clin Pharmacol. 1996;41(1):7–12.
Hogger P, Rohdewald P. Comparison of tilidine/naloxone, tramadol and bromfenac in experimental pain: a double-blind randomized crossover study in healthy human volunteers. Int J Clin Pharmacol Therap. 1999;37(8):377–85.
Hummel T, Roscher S, Pauli E, Frank M, Liefhold J, Fleischer W, et al. Assessment of analgesia in man: tramadol controlled release formula vs. tramadol standard formulation. Eur J Clin Pharmacol. 1996;51(1):31–8.
Thurauf N, Fleischer WK, Liefhold J, Schmid O, Kobal G. Dose dependent time course of the analgesic effect of a sustained-release preparation of tramadol on experimental phasic and tonic pain. Br J Clin Pharmacol. 1996;41(2):115–23.
Sarbu A, Radulescu F, Robertson S, Bouchard S. Onset of analgesic effect and plasma levels of controlled-release tramadol (Tramadol Contramid once-a-day) 200-mg tablets in patients with acute low back pain. J Opioid Manag. 2008;4(5):285–92.
Olesen AE, Andresen T, Staahl C, Drewes AM. Human experimental pain models for assessing the therapeutic efficacy of analgesic drugs. Pharmacol Rev. 2012;64(3):722–79.
Luginbuhl M, Schnider TW, Petersen-Felix S, Arendt-Nielsen L, Zbinden AM. Comparison of five experimental pain tests to measure analgesic effects of alfentanil. Anesthesiology. 2001;95(1):22–9.
Gustorff B, Hoerauf KH, Lierz P, Kress HG. Comparison of different quantitative sensory testing methods during remifentanil infusion in volunteers. Br J Anaesth. 2003;91(2):203–8.
Skinner-Robertson S, Mouksassi MS, Varin F. Evaluation of an experimental pain model by noncompartmental analysis. Pain Physician. 2018;21:363–72.
Kastrissios H, Walker JR, Carrothers TJ, Kshirsagar S, Khariton T, Habtemariam B, et al. Population pharmacokinetic model for a novel oral hypoglycemic formed in vivo: comparing the use of active metabolite data alone versus using data of upstream and downstream metabolites. J Clin Pharmacol. 2012;52(3):404–15.
Mould DR, Upton RN. Basic concepts in population modeling, simulation, and model-based drug development-part 2: introduction to pharmacokinetic modeling methods. CPT Pharmacometrics Syst Pharmacol. 2013;2:e38.
Skinner-Robertson S, Fradette C, Bouchard S, Mouksassi MS, Varin F. Pharmacokinetics of tramadol and O-desmethyltramadol enantiomers following administration of extended-release tablets to elderly and young subjects. Drugs Aging. 2015;32(12):1029–43.
Research FaDACfDE. Guidance for industry: Bioanalytical method validation. FDA Maryland; 2001.
Tzvetkov MV, Saadatmand AR, Lötsch J, Tegeder I, Stingl JC, Brockmöller J. Genetically polymorphic OCT1: another piece in the puzzle of the variable pharmacokinetics and pharmacodynamics of the opiodergic drug tramadol. Clin Pharmacol Ther. 2011;90(1):143–150.
Nies AT, Koepsell H, Winter S, Burk O, Klein K, Kerb R, Zanger U, Keppler D, Schwab M, Schaeffeler E. Expression of organic cation transporters OCT1 (SLC22A1) and OCT3 (SLC22A3) is affected by genetic factors and cholestasis in human liver. Hepatology 2009;50(4):1227–1240.
Katims JJ. Electrodiagnostic functional sensory evaluation of the patient with pain: a review of the neuroselective current perception threshold and pain tolerance threshold. Pain Digest. 1998;8:219–30.
Hutmacher MM, Kowalski KG. Covariate selection in pharmacometric analyses: a review of methods. Br J Clin Pharmacol. 2015;79(1):132–47.
Sauerbrei W, Schumacher M. A bootstrap resampling procedure for model building: application to the Cox regression model. Stat Med. 1992;11(16):2093–109.
Kemp J, Despres O, Pebayle T, Dufour A. Differences in age-related effects on myelinated and unmyelinated peripheral fibres: a sensitivity and evoked potentials study. Eur J Pain. 2014;18(4):482–8.
Verdu E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst. 2000;5(4):191–208.
Tseng MT, Chiang MC, Yazhuo K, Chao CC, Tseng WY, Hsieh ST. Effect of aging on the cerebral processing of thermal pain in the human brain. Pain. 2013;154(10):2120–9.
Brouquet A, Cudennec T, Benoist S, Moulias S, Beauchet A, Penna C, et al. Impaired mobility, ASA status and administration of tramadol are risk factors for postoperative delirium in patients aged 75 years or more after major abdominal surgery. Ann Surg. 2010;251(4):759–65.
Noble M, Tregear SJ, Treadwell JR, Schoelles K. Long-term opioid therapy for chronic noncancer pain: a systematic review and meta-analysis of efficacy and safety. J Pain Symptom Manag. 2008;35(2):214–28.
(CDER). CfDE. Guidance for industry: Pharmacokinetics in patients with renal impairment: Study design, data analysis and impact on dosing and labelling. In: Administration UsDoHaHSFaD, editor. Maryland 2010.
Acknowledgements
MDS Pharma conducted the clinical portions of the pharmacokinetics study. Dr Chunlin Chen is thanked for his participation in the collection of the pharmacodynamic data.
Author information
Authors and Affiliations
Contributions
The pharmacodynamic measures and analyses presented herein were conducted entirely independently as part of the doctoral research of SSR, PhD, who also managed the literature searches and summaries of previous related work and wrote the first draft of the manuscript. FV, PhD and M-SM, PhD provided revisions for intellectual content and final approval of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The study was contracted by Labopharm Inc. Sybil Skinner-Robertson was an employee of Labopharm Inc. prior to Nov 2011. France Varin and Mohamad-Samer Mouksassi have no conflicts of interest.
Funding
Labopharm sponsored the conduct of the study but not the analyses presented herein.
Ethical approval and informed consent
Before initiation of the study, the protocol and informed consent for this study were reviewed and approved by two independent ethics committees (Comité d’Ethique de la Recherche des Sciences de la Santé, Université de Montréal; and Investigational Review Board, MDS Pharma Services, Montreal). All subjects provided their written informed consent prior to the initiation of any study-related procedures. The study was conducted in accordance with the Declaration of Helsinki as well as the Enoncé de politique des trois Conseils. The study is registered at clinicaltrials.gov (NCT02329561).
Rights and permissions
About this article
Cite this article
Robertson, S.S., Mouksassi, M.S. & Varin, F. Population Pharmacokinetic/Pharmacodynamic Modeling of O-Desmethyltramadol in Young and Elderly Healthy Volunteers. Drugs Aging 36, 747–758 (2019). https://doi.org/10.1007/s40266-019-00681-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-019-00681-w