Abstract
Background
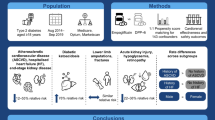
Clopidogrel has been widely used to prevent atherothrombotic events. Since 2011, pharmacists have offered their patients the opportunity to switch to generic clopidogrel, an economic alternative. Whether bioequivalence of generic cardiovascular drugs translates into clinical equivalence at a population level remains unclear and needs to be further documented.
Objective
We aimed to evaluate the impact of generic clopidogrel commercialization on adverse events (AEs): hospitalizations or emergency room (ER) consultations.
Methods
This is an interrupted time series analysis using the Quebec Integrated Chronic Disease Surveillance System. We included all patients ≥ 66 years old who were users of the brand-name clopidogrel or a generic version (n = 6) 24 months before and up to 12 months after generics commercialization. Rates of AEs were computed, and periods before and after generics commercialization were analyzed by segmented regression models along with exploratory analyses (generic vs. brand name). Sensitivity analyses were also performed using stratification of the time series by (1) sex, (2) the number of prevalent cardiovascular comorbidities, and (3) socioeconomic status.
Results
Time series were constituted of 89,525 clopidogrel users (mean age 78 years, 45% women, 71% ischemic heart disease, 34% stroke). For all users, there was a mean rate of 157 AEs per 1000 user-months, stable trend before (−0.1% [95% confidence interval −0.3 to 0.1] and after (0.0% [− 0.5 to 0.6]) generics commercialization. In exploratory analyses, once generic clopidogrel versions were commercialized, rates of AEs were 19.2% (95% CI 11.7–26.7) higher for generic versus brand-name users. This difference persisted up to 1 year. Sensitivity analyses yielded similar results.
Conclusions
The population treated with clopidogrel had similar rates of hospitalizations or ER consultations before and after generics commercialization. However, differences in rates of hospitalizations or ER consultations between generic and brand-name clopidogrel users may represent a drug safety signal which remains to be validated. Using a different study design, permitting adjustment for potential confounders, could be useful in this regard.




Similar content being viewed by others
References
United States Patent and Trademark Office. General information concerning patents. https://www.uspto.gov/patents-getting-started/general-information-concerning-patents. Accessed 21 Nov 2018.
Office de la propriété intellectuelle du Canada. Base de données sur les brevets canadiens. http://brevets-patents.ic.gc.ca/opic-cipo/cpd/fra/aide/contenu/aide_informations_generales.html. Accessed 3 Apr 2015.
European Patent Office. Biotechnology patents at the EPO. http://www.epo.org/news-issues/issues/biotechnology-patents.html. Accessed 28 Aug 2017.
Leclerc J. Surveillance des consultations à l’urgence et des hospitalisations chez les utilisateurs de médicaments génériques et originaux en cardiologie, au Québec. 2017; Université Laval (448 pages).
Sanofi-Aventis Canada Inc. Monographie de produit Plavix (clopidogrel). http://products.sanofi.ca/fr/plavix.pdf. Accessed 20 Oct 2016.
Leclerc J, Blais C, Guénette L, et al. Médicaments génériques et médicaments originaux: faire la différence. Perspect Infirmière. 2016;13:39–46.
Santé Canada. Ligne directrice—Normes en matière d’études de biodisponibilités comparatives: Formes pharmaceutiques de médicaments à effets systémiques. https://www.canada.ca/fr/sante-canada/services/medicaments-produits-sante/medicaments/demandes-presentations/lignes-directrices/biodisponibilite-bioequivalence/normes-matiere-etudes-biodisponibilite-comparatives-formes-pharmaceutiques-medicaments-effets-systemiques.html. Accessed 29 Oct 2018.
Jackevicius C, Tu JV, Krumholz HM, et al. Comparative effectiveness of generic atorvastatin and Lipitor(R) in patients hospitalized with an acute coronary syndrome. J Am Heart Assoc. 2016;5(4):e003350.
Caldeira D, Fernandes RM, Costa J, et al. Branded versus generic clopidogrel in cardiovascular diseases: a systematic review. J Cardiovasc Pharmacol. 2013;61:277–82.
Ko DT, Krumholz HM, Tu JV, et al. Clinical outcomes of Plavix and generic clopidogrel for patients hospitalized with an acute coronary syndrome. Circ Cardiovasc Qual Outcomes. 2018;11:e004194.
Leclerc J, Blais C, Rochette L, et al. Impact of the commercialization of three generic angiotensin II receptor blockers on adverse events in Quebec, Canada: a population-based time series analysis. Circ Cardiovasc Qual Outcomes. 2017;10:1–9.
Stoupakis G, Teichholz L. Stent thrombosis after switch from nongeneric to generic clopidogrel. Int J Case Rep Images. 2014;5:41–4.
Leclerc J, Blais C, Rochette L, et al. Trends in hospital visits for generic and brand-name warfarin users in Quebec, Canada; a population-based time series analysis. Am J Cardiovasc Drugs. 2018;. https://doi.org/10.1007/s40256-018-0309-9.
Leclerc J. Recall of NDMA-contaminated pseudogeneric valsartan; best generics finally no better than others? Can J Cardiol. 2018;34(1370):e13.
Andy E. Sartan drug contamination brings cancer uncertainty. https://www.chemistryworld.com/news/sartan-drug-contamination-brings-cancer-uncertainty/3009849.article. Accessed 16 Jan 2019.
European Medicine Agency. Update on review of recalled valsartan medicines. http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2018/08/news_detail_003000.jsp&mid=WC0b01ac058004d5c1. Accessed Aug 6 2018.
Wagner AK, Soumerai SB, Zhang F, et al. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299–309.
Bernal JL, Cummins Steven, Gasparrini Antonio. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017;46:348–55.
Blais C, Jean S, Sirois C, et al. Quebec Integrated Chronic Disease Surveillance System (QICDSS), an innovative approach. Chron Dis Injur Can. 2014;34:226–35.
Statistics Canada. Population by year, by province and territory. http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/demo02a-eng.htm. Accessed 28 Aug 2017.
Machelle W, Tamblyn RM, Huang A. Validation of diagnostic codes within medical services claims. J Clin Epidemiol. 2004;57:131–41.
Lambert L, Blais C, Hamel D, et al. Evaluation of care and surveillance of cardiovascular disease: can we trust medico-administrative hospital data? Can J Cardiol. 2012;28:162–8.
Tambly R, Lavoie G, Petrella L, et al. The use of prescription claims databases in pharmacoepidemiological research: the accuracy and comprehensiveness of the prescription claims database in Quebec. J Clin Epidemiol. 1995;48:999–1009.
Apotex inc. Monographie de produit Apo-clopidogrel (clopidogrel). http://hc-sc.gc.ca/dhp-mps/prodpharma/databasdon/index-fra.php. Accessed 20 Oct 2016.
Mylan Pharmaceuticals. Monographie de produit Mylan-clopidogrel (clopidogrel). http://hc-sc.gc.ca/dhp-mps/prodpharma/databasdon/index-fra.php. Accessed 20 Oct 2016.
Actavis Pharma Company. Monographie de produit: Act clopidogrel (clopidogrel). http://hc-sc.gc.ca/dhp-mps/prodpharma/databasdon/index-fra.php. Accessed 20 Oct 2016.
Pharmascience Inc. Monographie de produit pms-clopidogrel (clopidogrel). http://hc-sc.gc.ca/dhp-mps/prodpharma/databasdon/index-fra.php. Accessed 20 Oct 2016.
Sandoz Canada Inc. Monographie de produit Sandoz clopidogrel (clopidogrel). https://www.sandoz.ca/en. Accessed 20 Oct 2016.
Teva Canada Limited. Monographie de produit Teva-clopidogrel (clopidogrel). Accessed 20 October 2016 at pdf.hres.ca
Dal Pan GJ, Lindquist M, Gelperin K. Chapter 7. Postmarketing spontaneous pharmacovigilance reporting systems. In: Strom BL, Kimmel SE, Hennessy S, editors. Textbook of Pharmacoepidemiology, 2nd edition West Sussex: Wiley; 2013: p 456.
World Health Organization. Pharmacovigilance. http://www.who.int/medicines/areas/quality_safety/safety_efficacy/pharmvigi/en/. Accessed 26 Sept 2017.
Gouvernement du Canada. Guidance document for industry—reporting adverse reactions to marketed health products. https://www.canada.ca/en/health-canada/services/drugs-health-products/reports-publications/medeffect-canada/guidance-document-industry-reporting-adverse-reactions-marketed-health-products-health-canada-2011.html. Accessed 26 Sept 2017.
Food and Drug Administration. Identifying safety signals by data mining the FDA adverse event reporting system with empirical signal. https://www.slideshare.net/perficientinc/identifying-safety-signals-by-data-mining-the-fda-adverse-event-reporting-system-with-empirica-signal-41514. Accessed 11 Oct 2017.
Pampalon R, Hamel D, Gamache P. A comparison of individual and area-based socio-economic data for monitoring social inequalities in health. Health Rep. 2009;20:85–94.
Diaz DA, Colgan ST, Langer Connie S, et al. Dissolution similarity requirements: how similar or dissimilar are the global regulatory expectations? Aaps J. 2016;18:15–22.
Paterson JM, Mamdani M, Juurlink DN, et al. Clinical consequences of generic warfarin substitution: an ecological study. JAMA. 2006;296:1969–72.
Almsherqi ZA, McLachlan CS, Sharef SM. Non-bleeding side effects of clopidogrel: have large multi-center clinical trials underestimated their incidence? Int J Cardiol. 2007;117:415–7.
Hellstrom Jorgen, Rudholm Niklas. Side effects of generic competition? Eur J Health Econ. 2004;5:203–8.
Pan CY, Chen CW, Xu CY, et al. Genetic polymorphisms and clopidogrel efficacy for acute ischemic stroke or transient ischemic attack: a systematic review and meta-analysis. Circulation. 2016;135(1):21–3.
Komosa A, Siller-Matula JM, Kowal J, et al. Comparison of the antiplatelet effect of two clopidogrel bisulfate formulations: plavix and generic-Egitromb. Platelets. 2015;26:43–7.
Ntalas IV, Kalantzi KI, Tsoumani ME, et al. Salts of clopidogrel: investigation to ensure clinical equivalence: a 12-month randomized clinical trial. J Cardiovasc Pharmacol Ther. 2016;21:516–25.
Institut national de santé publique du Québec. Vivre dans une collectivité rurale plutôt qu’en ville fait-il vraiment une différence en matière de santé et de bien-être?. https://www.inspq.qc.ca/pdf/publications/269-RuraliteVilleDifference.pdf. Accessed 7 May 2017.
Gagne J, Polinski J, Jiang W, et al. Outcomes associated with generic drugs approved using product-specific determinations of therapeutic equivalence. Drugs. 2017;77:427–33.
Acknowledgements
This study was conducted at the National Public Health Institute of Quebec (INSPQ) as part of the continuous chronic disease surveillance mandate granted by the provincial minister of health and social services. Jacinthe Leclerc is the recipient of a studentship from the program Ministère de l’Éducation et de l’Enseignement supérieur-Universités. Dr. Paul Poirier is a senior clinical researcher of the Fonds de recherche du Québec-Santé (FRQ-S). Dr. Line Guénette holds a Junior-1 clinical researcher salary award from the FRQ-S in partnership with the Société québécoise d’hypertension artérielle (SQHA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
There was no funding for this study, part of the continuous chronic disease surveillance mandate in the province of Quebec, Canada.
Conflict of interest
Paul Poirier has received honoraria for continuing medical education/consultant/expert events from Abbott Vascular, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Janssen, Merck, Novartis, NovoNordisk, Pfizer, Roche, Sanofi-Aventis, Servier and Valeant. The industry had no influence in any decisions made through this project. Jacinthe Leclerc, Claudia Blais, Louis Rochette, Denis Hamel and Line Guénette have no conflict of interest relevant to the content of this article.
Contributions of Authors
Protocol design: Jacinthe Leclerc, Paul Poirier, Claudia Blais, Line Guénette, Denis Hamel and Louis Rochette. Data acquisition, analysis: Louis Rochette, Denis Hamel and Jacinthe Leclerc. Interpretation of data: Jacinthe Leclerc, Paul Poirier, Claudia Blais, Line Guénette, Denis Hamel and Louis Rochette. Drafting of the work: Jacinthe Leclerc. Revisions of critically important intellectual content: Claudia Blais, Line Guénette, Denis Hamel, Louis Rochette and Paul Poirier. Final approval of the version to be published: Paul Poirier. Agreement to be accountable for all aspects of the work: Jacinthe Leclerc, Claudia Blais, Louis Rochette, Denis Hamel, Line Guénette and Paul Poirier.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Leclerc, J., Blais, C., Rochette, L. et al. Did Generic Clopidogrel Commercialization Affect Trends of ER Consultations and Hospitalizations in the Population Treated with Clopidogrel?. Drugs Aging 36, 759–768 (2019). https://doi.org/10.1007/s40266-019-00679-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-019-00679-4