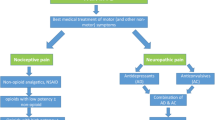
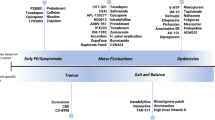
Abstract
The concept of personalised medicine in Parkinson’s disease has arrived where the implications of findings made in research are certain to have an increasing impact upon clinical practice. Disease heterogeneity in Parkinson’s disease has been well described and lends itself to the construct of personalised medicine where it is hypothesised that a greater understanding of genetic and pathophysiological contributions may underpin the sub-groups described. This in turn has driven the development of potentially individualised disease-modifying therapies where, for example, we are beginning to see treatments that target patients with Parkinson’s disease with specific genetic mutations. Furthermore, clinicians are increasingly recognising the need to tailor their management approach to patients depending on their age of presentation, acknowledging differential side-effect profiles and responses especially when considering the use of device-assisted technologies such as infusion or surgery. Clearly, individualising the treatment of both motor and non-motor symptoms will remain imperative but, in the future, personalised medicine may provide clearer insights into various aspects of a patient’s symptomatology, disease course and thus the best therapeutic approaches.




Similar content being viewed by others
References
Ginsburg GS, Willard HF. Genomic and personalized medicine: foundations and applications. Transl Res. 2009;154(6):277–87.
Obeso JA, Stamelou M, Goetz CG, et al. Past, present, and future of Parkinson’s disease: a special essay on the 200th anniversary of the shaking palsy. Mov Disord. 2017;32(9):1264–310.
Titova N, Padmakumar C, Lewis SJ, et al. Parkinson’s: a syndrome rather than a disease? J Neural Transm (Vienna). 2017;124(8):907–14.
Ehgoetz Martens KA, Shine JM, Walton CC, et al. Evidence for subtypes of freezing of gait in Parkinson’s disease. Mov Disord. 2018;33(7):1174–8.
Mu J, Chaudhuri KR, Bielza C, et al. Parkinson’s disease subtypes identified from cluster analysis of motor and non-motor symptoms. Front Aging Neurosci. 2017;9:301.
Lawton M, Baig F, Rolinski M, et al. Parkinson’s disease subtypes in the Oxford Parkinson Disease Centre (OPDC) discovery cohort. J Parkinsons Dis. 2015;5(2):269–79.
Lewis SJ, Foltynie T, Blackwell AD, et al. Heterogeneity of Parkinson’s disease in the early clinical stages using a data driven approach. J Neurol Neurosurg Psychiatry. 2005;76(3):343–8.
Erro R, Vitale C, Amboni M, et al. The heterogeneity of early Parkinson’s disease: a cluster analysis on newly diagnosed untreated patients. PLoS One. 2013;8(8):e70244.
Szeto JY, O’Callaghan C, Shine JM, et al. The relationships between mild cognitive impairment and phenotype in Parkinson’s disease. NPJ Parkinsons Dis. 2015;1:15015.
Mestre TA, Eberly S, Tanner C, et al. Reproducibility of data-driven Parkinson’s disease subtypes for clinical research. Parkinsonism Relat Disord. 2018. https://doi.org/10.1016/j.parkreldis.2018.07.009 (Epub ahead of print).
Selikhova M, Williams DR, Kempster PA, et al. A clinico-pathological study of subtypes in Parkinson’s disease. Brain. 2009;132(Pt 11):2947–57.
Fahn S, Jankovic J, Hallett M. Principles and practice of movement disorders. 2nd ed. Edinburgh: Elsevier/Saunders; 2011. p. 548 (vii).
Sulzer D, Alcalay RN, Garretti F, et al. T cells from patients with Parkinson’s disease recognize alpha-synuclein peptides. Nature. 2017;546(7660):656–61.
Dzamko N, Geczy CL, Halliday GM. Inflammation is genetically implicated in Parkinson’s disease. Neuroscience. 2015;302:89–102.
Alessi DR, Sammler E. LRRK2 kinase in Parkinson’s disease. Science. 2018;360(6384):36–7.
O’Donnell PH, Ratain MJ. Germline pharmacogenomics in oncology: decoding the patient for targeting therapy. Mol Oncol. 2012;6(2):251–9.
Atashrazm F, Hammond D, Perera G, et al. Reduced glucocerebrosidase activity in monocytes from patients with Parkinson’s disease. Sci Rep. 2018;8(1):15446.
Titova N, Chaudhuri KR. Personalized medicine and nonmotor symptoms in Parkinson’s disease. Int Rev Neurobiol. 2017;134:1257–81.
Nandipati S, Litvan I. Environmental exposures and Parkinson’s disease. Int J Environ Res Public Health. 2016;13(9):881. https://doi.org/10.3390/ijerph13090881.
Antonini A, Moro E, Godeiro C, et al. Medical and surgical management of advanced Parkinson’s disease. Mov Disord. 2018;33(6):900–8.
Giugni JC, Okun MS. Treatment of advanced Parkinson’s disease. Curr Opin Neurol. 2014;27(4):450–60.
Silberstein P, Bittar RG, Boyle R, et al. Deep brain stimulation for Parkinson’s disease: Australian referral guidelines. J Clin Neurosci. 2009;16(8):1001–8.
Trenkwalder C, Chaudhuri KR, Garcia Ruiz PJ, et al. Expert Consensus Group report on the use of apomorphine in the treatment of Parkinson’s disease: clinical practice recommendations. Parkinsonism Relat Disord. 2015;21(9):1023–30.
Fung VS. New and emerging treatments for Parkinson disease. Med J Aust. 2015;202(6):283–4.
Thenganatt MA, Jankovic J. Parkinson disease subtypes. JAMA Neurol. 2014;71(4):499–504.
Schrag A, Hovris A, Morley D, et al. Young- versus older-onset Parkinson’s disease: impact of disease and psychosocial consequences. Mov Disord. 2003;18(11):1250–6.
Calne SM, Kumar A. Young onset Parkinson’s disease: practical management of medical issues. Parkinsonism Relat Disord. 2008;14(2):133–42.
Seier M, Hiller A. Parkinson’s disease and pregnancy: an updated review. Parkinsonism Relat Disord. 2017;40:11–7.
Chen JJ, Swope DM, Dashtipour K. Comprehensive review of rasagiline, a second-generation monoamine oxidase inhibitor, for the treatment of Parkinson’s disease. Clin Ther. 2007;29(9):1825–49.
Weintraub D, David AS, Evans AH, et al. Clinical spectrum of impulse control disorders in Parkinson’s disease. Mov Disord. 2015;30(2):121–7.
Lenka A, Padmakumar C, Pal PK. Treatment of older Parkinson’s disease. Int Rev Neurobiol. 2017;132:381–405.
Lewis SJ, Gangadharan S, Padmakumar CP. Parkinson’s disease in the older patient. Clin Med (Lond). 2016;16(4):376–8.
Meng Y, Voisin MR, Suppiah S, et al. Is there a role for MR-guided focused ultrasound in Parkinson’s disease? Mov Disord. 2018;33(4):575–9.
Williams DR, Evans AH, Fung VS, et al. Practical approaches to commencing device-assisted therapies for Parkinson disease in Australia. Intern Med J. 2017;47(10):1107–13.
Lee JY, Lee EK, Park SS, et al. Association of DRD3 and GRIN2B with impulse control and related behaviors in Parkinson’s disease. Mov Disord. 2009;24(12):1803–10.
Lewis SJ. Disease-modifying approaches for Parkinson disease. Med J Aust. 2018;208(9):377–8.
Stebbins GT, Goetz CG, Burn DJ, et al. How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: comparison with the unified Parkinson’s disease rating scale. Mov Disord. 2013;28(5):668–70.
Klein C, Westenberger A. Genetics of Parkinson’s disease. Cold Spring Harb Perspect Med. 2012;2(1):a008888.
Pagano G, Ferrara F, Brooks DJ, et al. Age at onset and Parkinson disease phenotype. Neurology. 2016;86(15):1400–7.
Thaler A, Bregman N, Gurevich T, et al. Parkinson’s disease phenotype is influenced by the severity of the mutations in the GBA gene. Parkinsonism Relat Disord. 2018;55:45–9.
Ferreira M, Massano J. An updated review of Parkinson’s disease genetics and clinicopathological correlations. Acta Neurol Scand. 2017;135(3):273–84.
O’Regan G, deSouza RM, Balestrino R, et al. Glucocerebrosidase mutations in Parkinson disease. J Parkinsons Dis. 2017;7(3):411–22.
Nyholm D. Duodopa® treatment for advanced Parkinson’s disease: a review of efficacy and safety. Parkinsonism Relat Disord. 2012;18(8):916–29.
Katzenschlager R, Poewe W, Rascol O, et al. Apomorphine subcutaneous infusion in patients with Parkinson’s disease with persistent motor fluctuations (TOLEDO): a multicentre, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2018;17(9):749–59.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Lauren E. Ryden receives a salary from the University of Sydney, ForeFront group and is currently completing a Neurodegenerative Disease Fellowship at the Brain and Mind Centre, Sydney, NSW, Australia. Simon J.G. Lewis is supported by an NHMRC-ARC Dementia Fellowship (#1110414) and funding to ForeFront, a collaborative research group at the Brain and Mind Centre, University of Sydney, from the NHMRC programme (#1132524), Dementia Research Team (#1095127), NeuroSleep Centre of Research Excellence (#1060992) grants and a Sydney Research Excellence Initiative 2020 grant. No sources of funding were received for the preparation of this article.
Conflict of interest
Lauren E. Ryden and Simon J. G. Lewis have no conflicts of interest that are directly relevant to the contents of this article.
Rights and permissions
About this article
Cite this article
Ryden, L.E., Lewis, S.J.G. Parkinson’s Disease in the Era of Personalised Medicine: One Size Does Not Fit All. Drugs Aging 36, 103–113 (2019). https://doi.org/10.1007/s40266-018-0624-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-018-0624-5