Abstract
Background
With the aging of the hepatitis C virus (HCV)-infected patient cohort and the availability of highly effective and tolerable treatment regimens, an increasing number of elderly patients are now eligible for HCV therapy. This study investigated clinical and epidemiologic characteristics of elderly HCV-infected patients as well as the effectiveness and safety of available therapies.
Methods
Patients were enrolled into the German Hepatitis C Registry (DHC-R), a prospective, multicenter, real-world cohort study. Patients were treated at the discretion of the physician, and data were collected by a web-based system.
Results
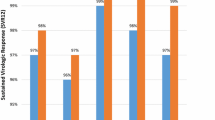
Of 7133 patients who initiated treatment, 686 (9.6%) were > 70 years of age. In patients > 70 years, intent-to-treat (ITT) SVR12 was 92.6% (514/555) compared to 90.7% (4521/4985) in patients ≤ 70 years of age. Overall, adverse events (AEs) were reported in 374 (54.5%) and 3435 patients (53.3%) > 70 or ≤ 70 years of age; 7.6% (52) and 3.6% (235) in the respective age groups had a serious AE. Twenty-two (3.2%) and 62 (1.0%) of the patients > 70 or ≤ 70 years discontinued treatment due to AEs. Death was reported in 34 patients, of whom eight were > 70 years of age. Frequent comorbidities in patients > 70 years of age were cardiac disease, renal disease and diabetes. Psychiatric disorders, substance abuse and viral co-infection were more frequent in younger patients.
Conclusion
Direct-acting antiviral therapies were well tolerated in patients older than 70 years. SVR12 rates in the elderly patient group were similar to those observed in younger patients. Differences in the prevalence of comorbidities between age groups warrant individualized attention with respect to drug–drug interactions and therapy adherence.
The study was registered in the German Clinical Trials Register, DRKS-ID: DRKS00009717.





Similar content being viewed by others
References
EASL. Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60(2):392–420.
Hepatitis C guidance. AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3):932–54.
Sarrazin C, Berg T, Buggisch P, Dollinger MM, Hinrichsen H, Hofer H, et al. Aktuelle Empfehlung zur Therapie der chronischen Hepatitis C. Z Gastroenterol. 2015;53(4):320–34.
Zeuzem S. Treatment options in Hepatitis C. Dtsch Arztebl Int. 2017;114(1–02):11–21.
Höner Zu Siederdissen C, Buggisch P, Böker K, Schott E, Klinker H, Pathil A, et al. Treatment of hepatitis C genotype 1 infection in Germany: effectiveness and safety of antiviral treatment in a real-world setting. United Eur Gastroenterol J. 2018;6(2):213–24. https://doi.org/10.1177/2050640617716607.
Buggisch P, Vermehren J, Mauss S, Günther R, Schott E, Pathil A et al. Real-world effectiveness of 8 weeks treatment with ledipasvir/sofosbuvir in chronic hepatitis C. J Hepatol 2017.
Welzel TM, Hinrichsen H, Sarrazin C, Buggisch P, Baumgarten A, Christensen S, et al. Real-world experience with the all-oral, interferon-free regimen of ombitasvir/paritaprevir/ritonavir and dasabuvir for the treatment of chronic hepatitis C virus infection in the German Hepatitis C Registry. J Viral Hepat. 2017;24(10):840–9.
Mücke MM, Mücke VT, Lange CM, Zeuzem S. Special populations: treating hepatitis C in patients with decompensated cirrhosis and/or advanced renal impairment. Liver Int. 2017;37(Suppl 1):19–25.
Rockstroh JK. Optimal therapy of HIV/HCV co-infected patients with direct acting antivirals. Liver Int. 2015;35(Suppl 1):51–5.
Vespasiani-Gentilucci U, Galati G, Gallo P, de Vincentis A, Riva E, Picardi A. Hepatitis C treatment in the elderly: new possibilities and controversies towards interferon-free regimens. World J Gastroenterol. 2015;21(24):7412–26.
Yang Z, Zhuang L, Yang L, Liu C, Lu Y, Xu Q, et al. Efficacy and safety of peginterferon plus ribavirin for patients aged ≥ 65 years with chronic hepatitis C: a systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2014;38(4):440–50.
Nudo CG, Wong P, Hilzenrat N, Deschênes M. Elderly patients are at greater risk of cytopenia during antiviral therapy for hepatitis C. Can J Gastroenterol. 2006;20(9):589–92.
Honda T, Katano Y, Shimizu J, Ishizu Y, Doizaki M, Hayashi K, et al. Efficacy of peginterferon-alpha-2b plus ribavirin in patients aged 65 years and older with chronic hepatitis C. Liver Int. 2010;30(4):527–37.
Poynard T, Ratziu V, Charlotte F, Goodman Z, McHutchison J, Albrecht J. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis c. J Hepatol. 2001;34(5):730–9.
Pradat P, Voirin N, Tillmann HL, Chevallier M, Trépo C. Progression to cirrhosis in hepatitis C patients: an age-dependent process. Liver Int. 2007;27(3):335–9.
Younossi Z, Park H, Henry L, Adeyemi A, Stepanova M. Extrahepatic manifestations of hepatitis C: a meta-analysis of prevalence, quality of life, and economic burden. Gastroenterology. 2016;150(7):1599–608.
van der Meer AJ, Berenguer M. Reversion of disease manifestations after HCV eradication. J Hepatol. 2016;65(1 Suppl):S95–108.
Hassoun Z, Willems B, Deslauriers J, Nguyen BN, Huet P. Assessment of fatigue in patients with chronic hepatitis C using the Fatigue Impact Scale. Dig Dis Sci. 2002;47(12):2674–81.
Reddy KR, Bourlière M, Sulkowski M, Omata M, Zeuzem S, Feld JJ, et al. Ledipasvir and sofosbuvir in patients with genotype 1 hepatitis C virus infection and compensated cirrhosis: an integrated safety and efficacy analysis. Hepatology. 2015;62(1):79–86.
Shiffman ML, Rustgi V, Bennett M, Forns X, Asselah T, Vila RP, et al. Corrigendum: safety and efficacy of ombitasvir/paritaprevir/ritonavir plus dasabuvir with or without ribavirin in HCV-infected patients taking concomitant acid-reducing agents. Am J Gastroenterol. 2016;111(7):1077.
Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370(21):1993–2001.
Welzel TM, Petersen J, Herzer K, Ferenci P, Gschwantler M, Wedemeyer H, et al. Daclatasvir plus sofosbuvir, with or without ribavirin, achieved high sustained virological response rates in patients with HCV infection and advanced liver disease in a real-world cohort. Gut. 2016;65(11):1861–70.
Leroy V, Angus P, Bronowicki J, Dore GJ, Hezode C, Pianko S, et al. Daclatasvir, sofosbuvir, and ribavirin for hepatitis C virus genotype 3 and advanced liver disease: a randomized phase III study (ALLY-3 +). Hepatology. 2016;63(5):1430–41.
Vermehren J, Peiffer K, Welsch C, Grammatikos G, Welker M, Weiler N, et al. The efficacy and safety of direct acting antiviral treatment and clinical significance of drug-drug interactions in elderly patients with chronic hepatitis C virus infection. Aliment Pharmacol Ther. 2016;44(8):856–65.
Conti F, Brillanti S, Buonfiglioli F, Vukotic R, Morelli MC, Lalanne C, et al. Safety and efficacy of direct-acting antivirals for the treatment of chronic hepatitis C in a real-world population aged 65 years and older. J Viral Hepat. 2017;24(6):454–63.
Toyoda H, Kumada T, Tada T, Shimada N, Takaguchi K, Senoh T, et al. Efficacy and tolerability of an IFN-free regimen with DCV/ASV for elderly patients infected with HCV genotype 1B. J Hepatol. 2017;66(3):521–7.
Akuta N, Sezaki H, Suzuki F, Kawamura Y, Hosaka T, Kobayashi M, et al. Favorable efficacy of daclatasvir plus asunaprevir in treatment of elderly Japanese patients infected with HCV genotype 1b aged 70 and older. J Med Virol. 2017;89(1):91–8.
Ogawa E, Furusyo N, Nomura H, Takahashi K, Higashi N, Kawano A, et al. Effectiveness and safety of sofosbuvir plus ribavirin for HCV genotype 2 patients 65 and over with or without cirrhosis. Antiviral Res. 2016;136:37–44.
Nishida N, Kono M, Minami T, Chishina H, Arizumi T, Takita M, et al. Safety, tolerability, and efficacy of sofosbuvir plus ribavirin in elderly patients infected with hepatitis C virus genotype 2. Dig Dis. 2016;34(6):632–9.
Lens S, Fernández I, Rodríguez-Tajes S, Hontangas V, Vergara M, Forné M et al. Interferon-free therapy in elderly patients with advanced liver disease. Am J Gastroenterol 2017.
Kartashev V, Döring M, Nieto L, Coletta E, Kaiser R, Sierra S. New findings in HCV genotype distribution in selected West European, Russian and Israeli regions. J Clin Virol. 2016;81:82–9.
Jacobson IM, Davis GL, El-Serag H, Negro F, Trépo C. Prevalence and challenges of liver diseases in patients with chronic hepatitis C virus infection. Clin Gastroenterol Hepatol. 2010;8(11):924–33.
Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. Lancet. 1997;349(9055):825–32.
Massard J, Ratziu V, Thabut D, Moussalli J, Lebray P, Benhamou Y, et al. Natural history and predictors of disease severity in chronic hepatitis C. J Hepatol. 2006;44(1 Suppl):S19–24.
Kiser JJ, Burton JR, Everson GT. Drug-drug interactions during antiviral therapy for chronic hepatitis C. Nat Rev Gastroenterol Hepatol. 2013;10(10):596–606.
Smolders EJ, Smit C, Tmm De Kanter C, Dofferhoff A, Arends JE, Brinkman K et al. High need to switch cART or co-medication with the initiation of DAAs in elderly HIV/HCV co-infected patients. J Acquir Immune Defic Syndr 2017.
Rice DP, Faragon JJ, Banks S, Chirch LM. HIV/HCV antiviral drug interactions in the era of direct-acting antivirals. J Clin Transl Hepatol. 2016;4(3):234–40.
Smolders EJ, de Kanter CTMM, de Knegt RJ, van der Valk M, Drenth JPH, Burger DM. Drug-drug interactions between direct-acting antivirals and psychoactive medications. Clin Pharmacokinet. 2016;55(12):1471–94.
Badri PS, King JR, Polepally AR, McGovern BH, Dutta S, Menon RM. Dosing recommendations for concomitant medications during 3D anti-HCV therapy. Clin Pharmacokinet. 2016;55(3):275–95.
Tacke F, Günther R, Buggisch P, Klinker H, Schober A, John C, et al. Treatment of HCV genotype 2 with sofosbuvir and ribavirin results in lower sustained virological response rates in real life than expected from clinical trials. Liver Int. 2017;37(2):205–11.
Cornberg M, Petersen J, Schober A, Mauss S, Böker KHW, Link R, et al. Real-world use, effectiveness and safety of anti-viral treatment in chronic hepatitis C genotype 3 infection. Aliment Pharmacol Ther. 2017;45(5):688–700.
Wiese M, Fischer J, Löbermann M, Göbel U, Grüngreiff K, Güthoff W, et al. Evaluation of liver disease progression in the German hepatitis C virus (1b)-contaminated anti-D cohort at 35 years after infection. Hepatology. 2014;59(1):49–57.
Fabrizi F, Verdesca S, Messa P, Martin P. Hepatitis C virus infection increases the risk of developing chronic kidney disease: a systematic review and meta-analysis. Dig Dis Sci. 2015;60(12):3801–13.
Younossi Z, Henry L. Systematic review: patient-reported outcomes in chronic hepatitis C—the impact of liver disease and new treatment regimens. Aliment Pharmacol Ther. 2015;41(6):497–520.
Acknowledgements
Data acquisition was conducted by DHC-R investigators. All authors made substantial contributions to the design of the analysis, writing, and/or interpretation of data in this publication. All authors critically revised the manuscript and approved this publication. All authors had access to all relevant data. Data were derived from the German Hepatitis C Registry (Deutsches Hepatitis C Register), a project of the German Liver Foundation (Deutsche Leberstiftung), managed by Leberstiftungs-GmbH Deutschland in cooperation with the Association of German Gastroenterologists in Private Practice (bng), with financial support from the German Center for Infection Research (DZIF) and the companies AbbVie Deutschland GmbH & Co. KG, Bristol-Myers Squibb GmbH & Co. KGaA, Gilead Sciences GmbH, Janssen-Cilag GmbH, MSD Sharp & Dohme GmbH as well as Roche Pharma AG (financial support until 2017-07-14). We thank all DHC-R investigators, study nurses and Leberstiftungs-GmbH Deutschland, in particular, Bianka Wiebner and Dr. Yvonne Serfert. Statistical analysis support was provided by Heike Pfeiffer-Vornkahl from e.factum GmbH (Butzbach, Germany).
Collaborators: Rainer Günther, Holger Hinrichsen, Renate Heyne, Johannes Roth, Tobias Goeser, Rainer Ullrich, Christine John, Wolf Peter Hofmann, Gerlinde Teuber, Hjördis Möller, Axel Baumgarten, Jeannette Schwenzer, Anita Pathil, Michael R. Kraus, Andreas Weber, Maria-Christina Jung, Guido Gerken, Christoph Antoni, Margareta Frank Doss, Andreas Schober, Martin Hoffstadt, Armand v. Lucadou, Hermann Steffens, Hartwig Klinker, Andreas Geier, Gerd Klausen, Peter Buggisch, Markus Cornberg, Christoph Sarrazin, Michael P. Manns, Claus Niederau, Ulla Protzer, Peter Schirmacher.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
GD: Speaker for AbbVie. TM: Consultancies: MERZ, AbbVie, MSD, Roche, BMS, Bayer, Intercept, and Gilead; speaker: MERZ; grants: Deutsche Forschungsgemeinschaft, Falk Foundation, and Intercept. JP: Grant/research support: BMS, Novartis, and Roche; clinical studies: AbbVie, Arrowhead, BMS, Eisai, Falk, Gilead, Hepatera, Hologic, Intercept, Janssen, Merck, MSD, Roche, Siemens, and Vertex; consultant/advisor: Abbott, AbbVie, Arrowhead, Assembly Pharma, BMS, Contravir, Gilead, GSK, Kedrion, Janssen, Merck, MSD, Novira, and Roche; sponsored lectures: Abbott, BMS, Boehringer, Gilead, Kedrion, Janssen, Merck, Merz, MSD, Novartis, and Roche. SM: Advisory committees or review panels: AbbVie, and MSD; speaker: AbbVie, Janssen, Gilead, and MSD. TZ: Received lecturer, consultant fees and/or travel support from AbbVie, BMS, Gilead, Janssen, MSD and Roche; grant: BMS. MM: Advisory committees: Bayer; speaker: Intercept and BMS; travel grants: AbbVie, Gilead, MSD, and Roche. KGS: Consultancies: AbbVie, Janssen, BMS, and MSD; speaker: Falk, AbbVie, Gilead, BMS, Janssen, Norgine, Merz, and MSD. TB: Speaker: AbbVie, Alexion, Bayer, BMS, Gilead, Intercept, Janssen, MSD/Merck, Merz, Novartis, Sirtex and Sequana Medical; grants: AbbVie, BMS, Gilead, Intercept, Janssen, MSD/Merck, Merz, Novartis, and Sequana Medical; adviser: AbbVie, Alexion, Bayer, BMS, Gilead, Intercept, Janssen, MSD/Merck, Merz, Novartis, and Sequana Medical. SZ: Consultancies/speaker for AbbVie, BMS, Gilead, Janssen, and Merck/MSD. DH: Advisor: Novartis, Gilead, BMS, Janssen, AbbVie, Roche, and MSD. KB: Speaker: MSD, Gilead, BMS and Roche. HW: Advisor: Falk, AbbVie, Novartis, Roche Diagnostics, Eiger, Janssen, GSK, Transgene, MSD, Roche, Gilead, Abbott, and BMS; consultancies: MyrGmbH; grants: Roche Diagnostics, Novartis, Gilead, Roche, Abbott, and MSD; speaker: AbbVie, Gilead, BMS, MSD, Novartis, and ITF. TMW: Consultancies/speaker for AbbVie, Bristol-Myers Squibb, Gilead, and Janssen.
Rights and permissions
About this article
Cite this article
Dultz, G., Müller, T., Petersen, J. et al. Effectiveness and Safety of Direct-Acting Antiviral Combination Therapies for Treatment of Hepatitis C Virus in Elderly Patients: Results from the German Hepatitis C Registry. Drugs Aging 35, 843–857 (2018). https://doi.org/10.1007/s40266-018-0572-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-018-0572-0