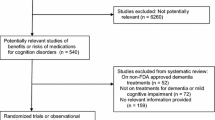
Abstract
The evidence base to guide withdrawal of antidementia medications in older people with dementia is limited; while some randomised controlled studies have considered discontinuation of cholinesterase inhibitors (ChEIs), no such studies examining discontinuation of the N-methyl-d-aspartate receptor antagonist memantine have been conducted to date. The purpose of this opinion article was to summarise the existing evidence on withdrawal of cholinesterase inhibitors and memantine, to highlight the key considerations for clinicians when making these prescribing decisions and to offer guidance as to when and how treatment might be discontinued. Until the evidence base is enhanced by the findings of large-scale, randomised controlled discontinuation trials of ChEIs and memantine that use multiple, clinically relevant, cognitive, functional and behavioural outcome measures, clinicians’ prescribing decisions involve balancing the risks of discontinuation with side effects and costs of continued treatment. Such decisions must be highly individualised and patient-centred.
Similar content being viewed by others
References
Alzheimer’s Disease International. World Alzheimer Report 2015: the global impact of dementia. An analysis of prevalence, incidence, cost and trends. Alzheimer’s Disease International (ADI), London; 2015.
Prince M, Bryce R, Albanese E, Wimo A, Ribiero W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9:63–75.
Raina P, Santaguida P, Ismaila A, Patterson C, Cowan D, Levine M, Booker L, Oremus M. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med. 2008;148(5):379–97.
Wong CW. Pharmacotherapy for dementia: a practical approach to the use of cholinesterase inhibitors and memantine. Drugs Aging. 2016. doi:10.1007/s40266-016-0372-3.
Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet. 1976;2(8000):1403.
Bartus RT, Dean RL 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–17.
Mauyqil T, Camicioli R. Systematic review and meta-analysis of combination therapy with cholinesterase inhibitors and memantine in Alzheimer’s disease and other dementias. Dement Geriatr Cogn Dis Extra. 2012;2:546–72.
Farlow MR, Grossberg GT, Sadowsky CH, Meng X, Somogyi M. A, 24-week, randomised, controlled trial of rivastigmine patch 13.3 mg/24 h versus 4.6 mg/34 h in severe Alzheimer’s dementia. CNS Neurosci Ther. 2013;19:745–52.
Voisin T, Vellas B. Diagnosis and treatment of patients with severe Alzheimer’s disease. Drugs Aging. 2009;26:135–44.
Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S, Mobius HJ. A 24-week open label extension study of memantine in moderate to severe Alzheimer disease. Arch Neurol. 2006;63:49–54.
Jain KK. Evaluation of memantine for neuroprotection in dementia. Expert Opin Investig Drugs. 2000;9:1397–406.
Cacabelos R, Takeda M, Winblad B. The glutamatergic system and neurodegeneration in dementia: preventive strategies in Alzheimer’s disease. Int J Geriatr Psychiatry. 1999;14:3–47.
Sucher NJ, Awobuluyi M, Choi YB, Lipton SA. NMDA receptors: from genes to channels. Trends Pharmacol Sci. 1996;17:348–55.
McShane R, Areosa Sastre A, Minakaran N. Memantine for dementia. Cochrane Database Syst Rev. 2006;(2):CD003154. doi:10.1002/14651858.CD003154.pub5.
Lanctôt KL, Rajaram RD, Herrmann N. Therapy for Alzheimer’s disease: how effective are current treatments? Ther Adv Neurol Disord. 2009;2(3):163–80.
Atri A, Rountree SD, Lopez OL, Doody RS. Validity, significance, strengths, limitations, and evidentiary value of real-world clinical data for combination therapy in Alzheimer’s disease: comparison of efficacy and effectiveness studies. Neurodegener Dis. 2012;10(1–4):170–4.
Rountree SD, Atri A, Lopez OL, Doody RS. Effectiveness of antidementia drugs in delaying Alzheimer’s disease progression. Alzheimers Dement. 2013;9(3):338–45.
Rountree SD, Chan W, Pavlik VN, Darby EF, Siddiqui S, Doody RS. Persistent treatment with cholinesterase inhibitors and/or memantine slows clinical progression of Alzheimer’s disease. Alzheimer Res Ther. 2009;1(2):7.
Wattmo C, Wallin A, Londos E, Minthon L. Long-term outcome and prediction models of activities of daily living in Alzheimer disease with cholinesterase inhibitor treatment. Alzheimer Dis Assoc Disord. 2011;25(1):63–72.
Birks JS. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev. 2006;(1):CD005593. doi:10.1002/14651858.CD005593.
Lanctôt KL, Herrmann N, Yau KK, Khan LR, Liu BA, LouLou MM, Einarson TR. Efficacy and safety of cholinesterase inhibitors in Alzheimer’s disease: a meta-analysis. CMAJ. 2003;169(6):557–64.
Stewart JT, Gorelik AR. Involuntary weight loss associated with cholinesterase inhibitors in dementia. J Am Geriatr Soc. 2006;54:1013.
Gillette-Guyonnet S, Cortes F, Cantet C, Vellas B. Long-term cholinergic treatment is not associated with greater risk of weight loss during Alzheimer’s disease: data from the French REAL.FR cohort. J Nutr Health Aging. 2005;9(2):69–73.
Droogsma E, van Asselt DZ, van Steijn JH, Schuur T, Huinink EJ. Effect of long-term treatment with galantamine on weight of patients with Alzheimer’s dementia. J Nutr Health Aging. 2013;17(5):461–5.
Hogan DB. Long-term efficacy and toxicity of cholinesterase inhibitors in the treatment of Alzheimer disease. Can J Psychiatry. 2014;59(12):618–23.
Thavorn K, Gomes T, Camacho X, Yao Z, Juurlink D, Mamdani M. Upper gastrointestinal bleeding in elderly adults with dementia receiving cholinesterase inhibitors: a population-based cohort study. J Am Geriatr Soc. 2014;62:382–4.
Gill SS, Anderson GM, Fischer HD, Bell CM, Li P, Normand SL, Rochon PA. Syncope and its consequences in patients with dementia receiving cholinesterase inhibitors: a population-based cohort study. Arch Intern Med. 2009;169(9):867–73.
Park-Wyllie LY, Mamdani MM, Li P, Gill SS, Laupacis A, Juurlink DN. Cholinesterase inhibitors and hospitalization for bradycardia: a population-based study. PLoS Med. 2009;6(9):e1000157.
Hernandez RK, Farwell W, Cantor MD, Lawler EV. Cholinesterase inhibitors and incidence of bradycardia in patients with dementia in the veterans affairs New England healthcare system. J Am Geriatr Soc. 2009;57(11):1997–2003.
Kim DH, Brown RT, Ding EL, Kiel DP, Berry SD. Dementia medications and risk of falls, syncope, and related adverse events: meta-analysis of randomized controlled trials. J Am Geriatr Soc. 2011;59(6):1019–31.
Howes LG. Cardiovascular effects of drugs used to treat Alzheimer’s disease. Drug Saf. 2014;37:391–5.
Rowland JP, Rigby J, Harper AC, Rowland R. Cardiovascular monitoring with acetylcholinesterase inhibitors: a clinical protocol. Adv Psychiatr Treat. 2007;13:178–84.
Helou R, Rhalimi M. Cholinesterase inhibitors and the risk of pulmonary disorders in hospitalized dementia patients. J Popul Ther Clin Pharmacol. 2010;17:e379–89.
Stephenson A, Seitz DP, Fischer HD, Gruneir A, Bell CM, Gershon AS, Fu L, Anderson GM, Austin PC, Rochon PA, Gill SS. Cholinesterase inhibitors and adverse pulmonary events in older people with chronic obstructive pulmonary disease and concomitant dementia: a population-based, cohort study. J Am Geriatr Soc. 2014;62:382–4.
Zannas AS, Okuno Y, Doraiswamy PM. Cholinesterase inhibitors and Pisa syndrome: a pharmacovigilance study. Pharmacotherapy. 2014;34:272–8.
Mansour D, Wong R, Kuskowski M, Dysken M. Discontinuation of acetylcholinesterase inhibitor treatment in the nursing home. Am J Geriatr Pharmacother. 2001;9:345–50.
Parsons C, Hughes CM, Passmore AP, Lapane KL. Withholding, discontinuing and withdrawing medications in dementia patients at the end of life: a neglected problem in the disadvantaged dying? Drugs Aging. 2010;27(6):435–49.
Parsons C, McCorry N, Murphy K, Byrne S, O’Sullivan D, O’Mahony D. Assessment of factors that influence physician decision-making regarding medication use in patients with dementia at the end of life. Int J Ger Psychiatry. 2014;29(3):281–90.
Qaseem A, Snow V, Cross JY, Forciea MA, Hopkins R, Shekelle P, Adelman A, Mehr D, Schellhase K, Campos-Outcalt D, Santaguida P, Owens DK. Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2008;148(5):370–8.
National Institute for Health and Care Excellence (NICE). Donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer’s disease. London: National Institute for Health and Care Excellence (NICE); 2011.
Deardorff WJ, Feen E, Grossberg GT. The use of cholinesterase inhibitors across all stages of Alzheimer’s disease. Drugs Aging. 2015;32:537–47.
National Collaborating Centre for Mental Health (UK). Dementia: a NICE-SCIE guideline on supporting people with dementia and their carers in health and social care. UK: The British Psychological Society & The Royal College of Psychiatrists; 2007.
Herrmann N, Lanctôt KL, Hogan DB. Pharmacological recommendations for the symptomatic treatment of dementia. The Canadian consensus conference on the diagnosis and treatment of dementia 2012. Alzheimer Res Ther. 2013;5:s5.
Moore A, Patterson C, Lee L, Vedel I, Bergman H. Fourth Canadian consensus conference on the diagnosis and treatment of dementia: recommendations for family physicians. Can Fam Physician. 2014;60:433–8.
Herrmann N, O’Regan J, Ruthirakuhan M, Kiss A, Eryavec G, Williams E, Lanctôt KL. A randomized placebo-controlled discontinuation study of cholinesterase inhibitors in institutionalized patients with moderate to severe Alzheimer’s disease. J Am Med Dir Assoc. 2016;17:142–7.
Singh S, Dudley C. Discontinuation syndrome following donepezil cessation. Int J Geriatr Psychiatry. 2003;18:282–4.
Kwak YT, Han IW, Suk SH, Koo MS. Two cases of discontinuation syndrome following cessation of memantine. Geriatr Gerontol Int. 2009;9:203–5.
Puangthong U, Hsiung GR. Critical appraisal of the long-term impact of memantine in treatment of moderate to severe Alzheimer’s disease. Neuropsychiatr Dis Treat. 2009;5:553–61.
Wilcock GK, Ballard CG, Cooper JA, Loft H. Memantine for agitation/aggression and psychosis in moderately severe to severe Alzheimer’s disease: a pooled analysis of 3 studies. J Clin Psychiatry. 2008;69(3):341–8.
Förstl H, Stamouli SS, Janetzky W, Galanopoulos A, Karageorgiou C, Tzanakaki M. Memantine in everyday clinical practice: a comparison of studies in Germany and Greece. Dement Geriatr Cogn Disord. 2011;32(4):267–72.
Herrmann N, Cappell J, Eryavec GM, Lanctôt K. Changes in nursing burden following memantine for agitation and aggression in long-term care residents with moderate to severe Alzheimer’s disease: an open label pilot study. CNS Drugs. 2011;25(5):425–33.
Rainer M, Wuschitz A, Jagsch C, Erb C, Chirikdjian JJ, Mucke HAM. Memantine in moderate to severe Alzheimer’s disease: an observational post-marketing study. J Neural Transm. 2011;118(8):1255–9.
Schulz JB, Rainer M, Klünemann HH, Kurz A, Wolf S, Sternberg K, Tennigkeit F. Sustained effects of once-daily memantine treatment on congition and functional communication skills in patients with moderate to severe Alzheimer’s disease: results of a 16-week open-label trial. J Alzheimers Dis. 2011;25(3):463–75.
Fox C, Crugel M, Maidment I, Auestad BH, Coulton S, Treloar A, Ballard C, Boustani M, Katona C, Livingston G. Efficacy of memantine for agitation in Alzheimer’s dementia: a randomised double-blind placebo controlled trial. PLoS One. 2012;7(5):e35185.
Grossberg GT, Manes F, Allegri RF, Gutierrez-Robledo LM, Gloger S, Xie L, Jia XD, Pejovic V, Miller ML, Perhach JL, Graham SM. The safety, tollerability, and efficacy of once-daily memantine (28 mg): a multinational, randomized, double-blind, placebo-controlled trial in patients with moderate-to-severe Alzheimer’s disease taking cholinesterase inhibitors. CNS Drugs. 2013;27:469–78.
Sinforiani E, Pasotti C, Chiapella L, Malinverni P, Zucchella C. Memantine in Alzhiemer’s disease: experience in an Alzheimer’s disease assessment unit. Aging Clin Exp Res. 2012;24(2):193–6.
Herrmann N, Gauthier S. Management of severe Alzheimer disease. CMAJ. 2008;179(12):1279–87.
Savic A, Mimica N. Two cases of loss of consciousness after long-term memantine treatment. J Am Med Dir Assoc. 2013;14:375–6.
Holmes C, Wilkinson D, Dean C, Vethanayagam S, Olivieri S, Langley A, Pandita-Gunawardena ND, Hogg F, Clare C, Damms J. The efficacy of donepezil in the treatment of neuropsychiatric symptoms in Alzheimer disease. Neurology. 2004;63:214–9.
Johannsen P, Salmon E, Hampel H, Xu Y, Richardson S, Qvitzau S, Schindler R. Assessing therapeutic efficacy in a progressive disease. CNS Drugs. 2006;20(4):311–25.
Howard R, McShane R, Lindesay J, Ritchie C, Baldwin A, Barber R, Burns A, Dening T, Findlay D, Holmes C, Hughes A, Jacoby R, Jones R, Jones R, McKeith I, Macharouthu A, O’Brien J, Passmore P, Sheehan B, Juszczak E, Katona C, Hills R, Knapp M, Ballard C, Brown R, Banerjee S, Onions C, Griffin M, Adams J, Gray R, Johnson T, Bentham P, Phillips P. Donepezil and memantine for moderate-to-severe Alzheimer’s disease. NEJM. 2012;366(10):893–903.
Howard R, McShane R, Lindesay J, Ritchie C, Baldwin A, Barber R, Burns A, Dening T, Findlay D, Holmes C, Jones R, McKeith I, Macharouthu A, O’Brien J, Sheehan B, Juszczak E, Katona C, Hills R, Knapp M, Ballard C, Brown RG, Banerjee S, Adams J, Johnson T, Bentham P, Phillips PJ. Nursing home placement in the donepezil and memantine in moderate to severe alzheimer’s disease (DOMINO-AD) trial: secondary and post-hoc analyses. Lancet Neurol. 2015;14:1171–81.
Gaudig M, Richarz U, Han J, Van Baelen B, Schäuble B. Effects of galantamine in Alzheimer’s disease: double-blind withdrawal studies evaluating sustained versus interrupted treatment. Curr Alzheimer Res. 2011;8:771–80.
Scarpini E, Bruno G, Zappala G, Adam M, Richarz U, Gaudig M, Jacobs A, Schäuble B. Cessation versus continuation of galantamine treatment after 12 months of therapy in patients with Alzheimer’s disease: a randomized, double blind, placebo controlled withdrawal trial. J Alzheimers Dis. 2011;26:211–20.
Tariot PN, Solomon PR, Morris JC, Kershaw P, Lilienfield S, Ding C. A, 5-month, randomized, placebo-controlled trial of galantamine in AD. The Galantamine USA-10 Study Group. Neurology. 2000;54:2269–76.
Rockwood K, Mintzer J, Truyen L, Wessel T, Wilkinson D. Effects of a flexible galantamine dose in Alzheimer’s disease: a randomized, controlled trial. J Neurol Neurosurg Psychiatry. 2001;71:589–95.
Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141:1356–64.
Galasko DR, Schmitt FA, Thomas R, Jin S, Bennett D, Ferris S. Detailed assessment of activities of daily living in moderate to severe Alzheimer’s disease. J Int Neuropsychol Soc. 2005;11:446–53.
Marin RS, Firinciogullari S, Biedrzycki RC. Group differences in the relationship between apathy and depression. J Nerv Ment Dis. 1994;182:235–9.
Bucks RS, Ashworth DL, Wilcock GK, Siegfried K. Assessment of activities of daily living in dementia: development of the Bristol Activities of Daily Living Scale. Age Ageing. 1996;25:113–20.
Guy W. CGI: Clinical Global Impressions. In: ECDEU Assessment manual for psychopharmacology. Rockville: US Department of Health, Education and Welfare, ADAMHA, MIMH Psychopharmacology Research Branch; 1976. p. 218–222.
Reisberg B, Ferris SH. Clinician’s interview-based impression of change-plus. In: Kelly C, Newton-Howes G, editors. Guide to assessment scales in dementia. London: Science; 1994.
Cohen-Mansfield J, Marx MS, Rosenthal AS. A description of agitation in a nursing home. J Geront. 1988;44(3):M77–84.
Gelinas I, Gauthier L, McIntyre M, Gauthier S. Development of a functional measure for persons with Alzheimer’s disease: the disability assessment for dementia. Am J Occupat Ther. 1999;53:471–81.
Smith SC, Lamping DL, Banerjee S, Harwood R, Foley B, Smith P, Cook JC, Murray J, Prince M, Levin E, Mann A, Knapp M. Measurement of health-related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluaion of current methodology. Health Technol Assess. 2005;9(10):1–93.
Goldberg DP, Gater R, Sartorius N, Ustun TB, Piccinelli M, Gureje O, Rutter C. The validity of two versions of the GHQ in the WHO study of mental illness in general health care. Psychol Med. 1997;27:191–7.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98.
Cummings JL, Donohue JA, Brooks RL. The relationship between donepezil and behavioral disturbances in patients with Alzheimer’s disease. Am J Geriatr Psychiatry. 2000;8:134–40.
Weiner MF, Martin-Cook K, Svetlik DA, Saine K, Foster B, Fontaine CS. The quality of life in late-stage dementia (QUALID) scale. J Am Med Dir Assoc. 2000;1(3):114–6.
Saxton J, McGonigle-Gibson K, Swihart A, Miller M, Boller F. Assessment of the severely impaired patient: description and validation of a new neuropsychological test battery. Psychol Assessment. 1990;2:298–330.
Cummings J. Mini-mental state examination: norms, normals and numbers. JAMA. 1993;269:2420–1.
Burns A, Lawlor B, Craig S. Assessment scales in old age psychiatry. 2nd ed. London: Martin Dunitz; 2004.
Feldman HH, Pirttila T, Dartigues JF, Everitt B, Van Baelen B, Schwalen S. Treatment with galantamine and time to nursing home placement in Alzheimer’s disease patients with and without cerebrovascular disease. Int J Geriatr Psychiatry. 2009;24:479–88.
O’Regan J, Lanctôt KL, Mazereeuw G, Herrmann N. Cholinesterase inhibitor discontinuation in patients with Alzheimer’s disease: a meta-analysis of randomized controlled trials. J Clin Psychiatry. 2015;76(11):e1424–31.
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2014. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 19 Apr 2016.
Higgins JP, Altman DG, Gøtzsche PC, Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. BMJ. 2011;343:d5928.
Daiello LA, Ott BR, Lapane KL, Reinert SE, Machan JT, Dore DD. Effect of discontinuing cholinesterase inhibitor therapy on behavioral and mood symptoms in nursing home patients with dementia. Am J Geriatr Pharmacother. 2009;7:74–83.
Burrows AB, Morris JN, Simon SE, Hirdes JP, Phillips C. Development of a minimum data set-based depression rating scale for use in nursing homes. Age Ageing. 2000;29:165–72.
Martin LH, Fries BE, Smith TF. Development and psychometric properties of an assessment for persons with intellectual disability—the interRAI ID. J Pol Pract Int Disab. 2007;4:23–9.
Herrmann N, Black SE, Li A, Lanctôt KL. Discontinuing cholinesterase inhibitors: results of a survey of Canadian dementia experts. Int Psychogeriatr. 2011;23(4):539–45.
Farrell B, Tsang C, Raman-Wilms L, Irving H, Conklin J, Pottie K. What are priorities for deprescribing for elderly patients capturing the voice of practitioners: a modified Delphi process. PLoS One. 2015;10(4):e0122246.
Holmes HM, Sachs GA, Shega JW, Hougham GW, Coy Hayley D, Dale W. Integrating palliative medicine into the care of persons with advanced dementia: identifying appropriate medication use. J Am Geriatr Soc. 2008;56(7):1306–11.
Parsons C, McCann L, Passmore P, Hughes C. Development and application of medication appropriateness indicators for persons with advanced dementia: a feasibility study. Drugs Aging. 2015;32(1):67–77.
Kröger E, Wilchesky M, Marcotte M, Voyer P, Morin M, Champoux N, Monette J, Aubin M, Durand PJ, Verrault R, Arcand M. Medication use among nursing home residents with severe dementia: identifying categories of appropriateness and elements of a successful intervention. J Am Med Dir Assoc 2015;16:629.e1–17
Hanlon JT, Aspinall SL, Handler SM, Gellad WF, Stone RA, Semla TP, Pugh MJV, Dysken MW. Potentially suboptimal prescribing for older veteran nursing home patients with dementia. Ann Pharmacother. 2015;49(1):20–8.
Tjia J, Briesacher BA, Peterson D, Liu Q, Andrade SE, Mitchell SL. Use of medications of questionable benefit in advanced dementia. JAMA Intern Med. 2014;174(11):1763–71.
Colloca G, Tosato M, Vetrano DL, Topinkova E, Fualova D, Gindin J, van der Roest HG, Landi F, Liperoti R, Bernabei R, Onder G. Inappropriate drugs in elderly patients with severe cognitive impairment: results from the SHELTER study. PLOS One. 2012;7(10):e46669.
Mattis S. Dementia rating scale (DRS) professional manual. Odessa: Psychological Assessment Resources, Inc.; 1988.
Leroi I, Overshott R, Byrne EJ, Daniel E, Burns A. Randomized controlled trial of memantine in dementia associated with Parkinson’s disease. Mov Disord. 2009;24(8):1217–40.
Fillit H, Hofbauer RK, Setyawan J, Tourkodimitris S, Fridman M, Pejović V, Eder MH, Lyketsos C. Memantine discontinuation and the health status of nursing home residents with Alzheimer’s disease. J Am Med Dir Assoc. 2010;11:636–44.
Johannson C, Ballard C, Hansson O, Palmqvist S, Minthon L, Aarsland D, Londos E. Efficacy of memantine in PDD and DLB: an extension study including washout and open-label treatment. Int J Geriatr Psychiatry. 2011;26:206–13.
Rockwood K. For how long should we use symptomatic therapies to treat people with Alzheimer’s disease? Can J Psychiatry. 2014;59(12):615–7.
Shanks M, Kivipelto M, Bullock R, Lane R. Cholinesterase inhibition: is there evidence for disease-modifying effects? Curr Med Opin Res. 2009;25(10):2439–46.
Giacobini E. Do cholinesterase inhibitors have disease-modifying effects in Alzheimer’s disease? CNS Drugs. 2001;1(2):85–91.
Golde TE. Disease modifying therapy for AD? J Neurochem. 2006;99:689–707.
Seltzer B. Is long-term treatment of Alzheimer’s disease with cholinesterase inhibitor therapy justified? Drugs Aging. 2007;24:881–90.
Salomone S, Caraci F, Leggio GM, Fedotova J, Drago F. New pharmacological strategies for treatment of Alzheimer’s disease: focus on disease modifying drugs. Br J Clin Pharmacol. 2011;73(4):504–17.
Van Dam D, De Deyn PP. Cognitive evaluation of disease-modifying efficacy of Galantamine and Memantine in the APP23 model. Eur Neuropsychopharmacol. 2006;16:59–69.
Workgroup AGSCW. American Geriatrics Society identifies another five things that healthcare providers and patients should question. J Am Geriatr Soc. 2014;62:950–60.
Rockwood K, Fay S, Jarrett P, et al. Effect of galantamine on verbal repetition in AD: a secondary analysis of the VISTA trial. Neurology. 2007;7(14):1116–21.
Fillit HM, Doody RS, Binaso K. Recommendations for best practices in the treatment of Alzheimer’s disease in managed care. Am J Geriatr Pharmacother. 2006;4(Suppl A):S9–24.
Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45.
Lavan AH, Gallagher P. Predicting the risk of adverse drug reactions in older adults. Ther Adv Drug Saf. 2016;7(1):11–22.
Hogan DB, Bailey P, Black S, Carswell A, Chertkow H, Clarke B, Cohen C, Fisk JD, Forbes D, Man-Son-Hing M, Lanctôt K, Morgan D, Thorpe L. Diagnosis and treatment of dementia: 5. Nonpharmacologic and pharmacologic therapy for mild to moderate dementia. CMAJ. 2008;179:1019–26.
Wattmo C, Minthon L, Wallin AK. Mild versus moderate stages of Alzheimer’s disease: three-year outcomes in a routine clinical setting of cholinesterase inhibitor therapy. Alz Res Ther. 2016;8:7.
Franco-Marina F, Garcia-González JJ, Wagner-Echeagaray F, Gallo J, Ugalde O, Sánchez-García S, Espinel-Bermúdez C, Juárez-Cedillo T, Rodríguez MAV, García-Peña C. The mini-mental state examination revisited: ceiling and floor effects after score adjustment for educational level in an aging Mexican population. Int Psychogeriatr. 2010;22:72–81.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflict of interest
Carole Parsons declares that she has no conflicts of interest that might be relevant to the content of this manuscript.
Rights and permissions
About this article
Cite this article
Parsons, C. Withdrawal of Antidementia Drugs in Older People: Who, When and How?. Drugs Aging 33, 545–556 (2016). https://doi.org/10.1007/s40266-016-0384-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-016-0384-z