Abstract
Introduction
Immunosenescence makes the elderly more susceptible to influenza complications and less responsive to vaccination. An intradermal formulation (IDflu) is one of several strategies being investigated to increase the immunogenicity of influenza vaccines.
Objective
The overall goal of the study was to assess the safety and immunogenicity of IDflu compared with the intramuscular route (IMflu) in the elderly.
Methods
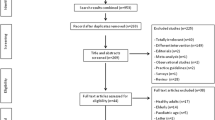
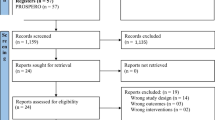
A meta-analysis of randomized controlled trials (RCTs) was performed. Included articles met the following criteria: RCTs; primary studies, not re-analyses or reviews; enrolment of elderly people; comparing the immunogenicity and/or safety of IDflu with IMflu; measuring seroprotection and/or seroconversion rate to assess immunogenicity; measuring local reactions and/or general symptoms and/or other mild local reactions that could affect acceptability of vaccine as safety indicators, according to the European Medicines Agency (EMA) criteria; published through January 2015.
Results
The results of our meta-analysis on seroprotection showed that IDflu is comparable to IMflu for each strain (A/H1N1: risk ratio [RR] 1.02, 95 % confidence interval [CI] 0.98–1.07; A/H3N2: RR 1.01, 95 % CI 0.99–1.04; B 1.02, 95 % CI 0.98–1.08). The seroconversion rate achieved with IDflu was comparable to that of the control group (A/H1N1: RR 1.08, 95 % CI 0.97–1.2; A/H3N2: RR 1.08, 95 % CI 0.96–1.21; B: RR 1.21, 95 % CI 1–1.45). Systemic reactogenicity appeared similar in the two groups, while local reactions were significantly more frequent in the IDflu group.
Conclusions
The novel IDflu appears to have the adequate balance between immunogenicity and safety in the elderly compared with IMflu, and its utilization may be considered among the possible strategies to enhance the control of seasonal influenza outbreaks according to the existing policy recommendations in the elderly.



Similar content being viewed by others
References
WHO. Global pandemic influenza action plan to increase vaccine supply: progress report 2006–2008. Geneva, 2009. http://whqlibdoc.who.int/hq/2009/WHO_IVB_09.05_eng.pdf. Accessed 20 June 2013.
Reed C, Kim IK, Singleton JA, et al. Estimated influenza illnesses and hospitalizations averted by vaccination—United Stated, 2013–14 influenza season. MMWR Morb Mortal Wkly Rep. 2014;12:1151–4.
ECDC. Seasonal influenza vaccination in Europe—Overview of vaccination recommendations and coverage rates in the EU Member States for the 2012–13 influenza season. Stockholm: ECDC. 2015. http://www.ecdc.europa.eu/en/publications/Publications/Seasonal-influenza-vaccination-Europe-2012-13.pdf. Accessed 30 Jan 2015.
Raphael D. Chapter 180. Influenza. In: Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL, Loscalzo J, editors. Harrison’s principles of internal medicine. 17th ed. New York: McGraw-Hill Co Inc; 2008. p. 1127–32.
Fiore AE, Uyeki TM, Broder K, et al. Centers for Disease Control and Prevention. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices (ACIP), 2010. MMWR Recomm Rep. 2010;59:1–62.
Villari P, Manzoli L, Boccia A. Methodological quality of studies and patient age as major sources of variation in efficacy estimates of influenza vaccination in healthy adults: a meta-analysis. Vaccine. 2004;22:3475–86.
Monto AS, Ansaldi F, Aspinall R, et al. Influenza control in the 21st century: optimizing protection of older adults. Vaccine. 2009;27:5043–53.
Parodi V, de Florentiis D, Martini M, et al. Inactivated influenza vaccines: recent progress and implications for the elderly. Drugs Aging. 2011;28:93–106.
Moher D, Liberati A, Tetzlaff J, et al. The PRISMA Group (2009): preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Jadad AR, Moore RA, Carrol D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clin Trials. 1996;17:1–12.
Chalmers TC, Smith H Jr, Blackburn B, et al. A method for assessing the quality of a randomized control trial. Controlled Clin Trials. 1981;2:31–49.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials. 1986;7:177–88.
Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48.
Higgins JP, Thompson SC. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–99.
Belshe RB, Newman FK, Cannon J, et al. Serum antibody responses after intradermal vaccination against influenza. N Engl J Med. 2004;351:2286–94.
Holland D, Booy R, De Looze F, et al. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: a randomized controlled trial. JID New Zealand. 2008;198:650–8.
Arnou R, Icardi G, De Decker M, et al. Intradermal influenza vaccine for older adults: a randomized controlled multicenter phase III study. Vaccine. 2009;27:7304–12.
Chi RC, Rock MT, Neuzil KM. Immunogenicity and safety of intradermal influenza vaccination in healthy older adults. CID. 2010;50:1331–8.
Chuaychoo B, Wongsurakiat P, Nana A, et al. The immunogenicity of intradermal influenza vaccination in COPD patients. Vaccine. 2010;28:4045–51.
Van Damme P, Arnou R, Kafeja F, et al. Evaluation of non-inferiority of intradermal versus adjuvanted seasonal influenza vaccine using two serological techniques: a randomized comparative study. BMC Infect Dis. 2010;10:134.
Ansaldi F, Orsi A, de Florentiis D, et al. Head-to-head comparison of an intradermal and a virosome influenza vaccine in patients over the age of 60: evaluation of immunogenicity, cross-protection, safety and tolerability. Hum Vaccin Immunother. 2013;9:591–8.
Hoon Han S, Hee Woo J, Weber F, et al. Immunogenicity and safety of Intanza®/IDflu® intradermal influenza vaccine in South Korean adults: a multicenter, randomized trial. Hum Vaccin Immunother. 2013;9:1971–7.
Scheifele DW, McNeil SA, Ward BJ, et al. Safety, immunogenicity, and tolerability of three influenza vaccines in older adults: results of a randomized, controlled comparison. Hum Vaccin Immunother. 2013;9:2460–73.
Tsang P, Gorse GJ, Strout CB, et al. Immunogenicity and safety of Fluzone® intradermal and high-dose influenza vaccines in older adults ≥65 years of age: a randomized, controlled, phase II trial. Vaccine. 2014;32:2507–17.
Della Cioppa G, Nicolay U, Lindert K, et al. A dose-ranging study in older adults to compare the safety and immunogenicity profiles of MF59®-adjuvanted and non-adjuvanted seasonal influenza vaccines following intradermal and intramuscular administration. Hum Vaccin Immunother. 2014;10:1701–10.
Camilloni B, Basileo M, Di Martino A, et al. Antibody responses to intradermal or intramuscular MF59-adjuvanted influenza vaccines as evaluated in elderly institutionalized volunteers during a season of partial mismatching between vaccine and circulating A(H3N2) strains. Immun Ageing. 2014;11:10.
Seo YB, Choi WS, Lee J, et al. Comparison of the immunogenicity and safety of the conventional subunit, MF59-adjuvanted, and intradermal influenza vaccines in the elderly. Clin Vaccine Immunol. 2014;21:989–96.
Chan TC, Hung IF, Chan KH, et al. Immunogenicity and safety of intradermal trivalent influenza vaccination in nursing home older adults: a randomized controlled trial. J Am Med Dir Assoc. 2014;15:607.e5–12.
Leroux-Roels I, Vets E, Freese R, et al. Seasonal influenza vaccine delivered by intradermal microinjection: a randomised controlled safety and immunogenicity trial in adults. Vaccine. 2008;26:6614–9.
Beran J, Ambrozaitis A, Laiskonis A, et al. Intradermal influenza vaccination of healthy adults using a new microinjection system: a 3-year randomised controlled safety and immunogenicity trial. BMC Med. 2009;2:7–13.
Arnou R, Eavis P, Pardo JR, et al. Immunogenicity, large scale safety and lot consistency of an intradermal influenza vaccine in adults aged 18–60 years: randomized, controlled, phase III trial. Hum Vaccin. 2010;6:346–54.
Marra F, Young F, Richardson K, et al. A meta-analysis of intradermal versus intramuscular influenza vaccines: immunogenicity and adverse events. Influenza Other Respir Viruses. 2013;7:584–603.
Manzoli L, De Vito C, Salanti G, et al. Meta-analysis of the immunogenicity and tolerability of pandemic influenza A 2009 (H1N1) vaccines. PLoS One. 2011;6:e24384.
Weniger BG, Papania MJ. Alternative vaccine delivery methods. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 5th ed. Philadelphia: Elsevier; 2008. p. 1357–92.
Reygrobellet C, Viala-Danten M, Meunier J, et al. Perception and acceptance of intradermal influenza vaccination: patient reported outcomes from phase 3 clinical trials. Hum Vaccin. 2010;6:336–45.
Foy JE, Hendriksz T, Malouf P, et al. Acceptability of fluzone intradermal vaccine to patients and vaccine administrators. J Am Osteopath Assoc. 2013;113:134–43.
Durando P, Alicino C, Alberti M, et al. Acceptance and safety of the intradermal influenza vaccine among the elderly in Italy: an on-field national study. Adv Ther. 2012;29:312–26.
Taddio A, Ipp M, Thivakaran S, et al. Survey of the prevalence of immunization on non-compliance due to needle fears in children and adults. Vaccine. 2012;30:4807–12.
Pileggi C, Carbone V, Pavia M, et al. Patients’ perceptions and related behaviours on role of primary care physician in Italy. Eur J Public Health. 2004;14:258–60.
Pavia M, Foresta MR, Carbone V, et al. Influenza and pneumococcal immunization in the elderly: knowledge, attitudes, and practices among general practitioners in Italy. Public Health. 2003;117:202–7.
Ansaldi F, Canepa P, Ceravolo A, et al. Intanza® 15 mcg intradermal influenza vaccine elicits cross-reactive antibody responses against heterologous A(H3N2) influenza viruses. Vaccine. 2012;30:2908–13.
Orsi A, Ansaldi F, de Florentiis D, et al. Cross-protection against drifted influenza viruses: options offered by adjuvanted and intradermal vaccines. Hum Vaccin Immunother. 2013;9:582–90.
Goronzy JJ, Weyand CM. T cell development and receptor diversity during aging. Curr Opin Immunol. 2005;17:468–75.
McElhaney JE. Influenza vaccine responses in older adults. Ageing Res Rev. 2011;10:379–88.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None.
Conflict of interest
C Pileggi, V Mascaro, A Bianco, CGA Nobile, and M Pavia declare they have no conflicts of interest relevant to the content of this article.
Rights and permissions
About this article
Cite this article
Pileggi, C., Mascaro, V., Bianco, A. et al. Immunogenicity and Safety of Intradermal Influenza Vaccine in the Elderly: A Meta-Analysis of Randomized Controlled Trials. Drugs Aging 32, 857–869 (2015). https://doi.org/10.1007/s40266-015-0303-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-015-0303-8