Abstract
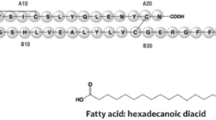
Insulin therapy is indispensable for achieving glycemic control in all patients with type 1 diabetes mellitus and many patients with type 2 diabetes mellitus. Insulin injections are associated with negative connotations in patients owing to administration discomfort and adverse effects such as hypoglycemia and weight gain. Insulin administered orally can overcome these limitations by providing a convenient and effective mode of delivery with a potentially lower risk of hypoglycemia. Oral insulin mimics the physiologic process of insulin secretion, absorption into the portal circulation, and subsequent peripheral delivery, unlike the subcutaneous route that results in peripheral hyperinsulinemia. Insulin tregopil (IN-105), a new generation human recombinant insulin, methoxy (polyethylene glycol) hexanoyl human recombinant insulin, is developed by Biocon as an ultra-fast onset short-acting oral insulin analog. This recombinant oral insulin is a single short-chain amphiphilic oligomer modified with the covalent attachment of methoxy-triethylene-glycol-propionyl moiety at Lys-β29-amino group of the B-chain via an amide linkage. Sodium caprate, an excipient in the insulin tregopil formulation, is a permeation enhancer that increases its absorption through the gastrointestinal tract. Also, meal composition has been shown to non-significantly affect its absorption. Several global randomized, controlled clinical trials have been conducted in type 1 and type 2 diabetes patients towards the clinical development of insulin tregopil. The formulation shows post-prandial glucose control that is more effective than placebo throughout the meal period; however, compared with an active comparator insulin aspart, the post-prandial control is more effective mainly in the early post-meal period. It shows a good safety profile with a lower incidence of clinically significant hypoglycemia. This review covers the overall clinical development of insulin tregopil establishing it as an ultra-fast onset, short-acting oral insulin analog for optimizing post-prandial glucose.






Similar content being viewed by others
References
Cheng R, Taleb N, Stainforth-Dubois M, Rabasa-Lhoret R. The promising future of insulin therapy in diabetes mellitus. Am J Physiol Endocrinol Metab. 2021;320:E886–90.
Dhapake PR, Chauriya CB, Umredkar RC. Painless insulin drug delivery systems—a review. Asian J Res Pharm Sci. 2017;7:1–7.
Korytkowski MT. In-patient management of diabetes: controversies and guidelines. Indian J Endocrinol Metab. 2013;17:630.
Russell-Jones D, Khan R. Insulin-associated weight gain in diabetes—causes, effects and coping strategies. Diabetes Obes Metab. 2007;9:799–812.
Swinnen SG, Hoekstra JB, DeVries JH. Insulin therapy for type 2 diabetes. Diabetes Care. 2009;32:S253–9.
Oramed, Ltd. A placebo-controlled, multi-center, randomized, phase 2b study to evaluate the efficacy and safety of ORMD-0801 in type 2 diabetes mellitus patients with inadequate glycemic control on oral therapy [Internet]. clinicaltrials.gov; 2020 Apr. Report No.: NCT03467932. https://clinicaltrials.gov/ct2/show/NCT03467932.
Muheem A, Shakeel F, Jahangir MA, Anwar M, Mallick N, Jain GK, et al. A review on the strategies for oral delivery of proteins and peptides and their clinical perspectives. Saudi Pharm J SPJ. 2016;24:413–28.
Wajcberg E, Miyazaki Y, Triplitt C, Cersosimo E, DeFronzo RA. Dose-response effect of a single administration of oral hexyl-insulin monoconjugate 2 in healthy nondiabetic subjects. Diabetes Care. 2004;27:2868–73.
Clement S, Dandona P, Still JG, Kosutic G. Oral modified insulin (HIM2) in patients with type 1 diabetes mellitus: results from a phase I/II clinical trial. Metabolism. 2004;53:54–8.
Kipnes M, Dandona P, Tripathy D, Still JG, Kosutic G. Control of postprandial plasma glucose by an oral insulin product (HIM2) in patients with type 2 diabetes. Diabetes Care. 2003;26:421–6.
Arbit E, Kidron M. Oral insulin delivery in a physiologic context: review. J Diabetes Sci Technol. 2017;11:825–32.
Sanlioglu AD, Altunbas HA, Balci MK, Griffith TS, Sanlioglu S. Clinical utility of insulin and insulin analogs. Islets. 2013;5:67–78.
Weisz B, Shrim A, Homko CJ, Schiff E, Epstein GS, Sivan E. One hour versus two hours postprandial glucose measurement in gestational diabetes: a prospective study. J Perinatol. 2005;25:241–4.
Association AD. Postprandial blood glucose. Diabetes Care. 2001;24:775–8.
Sivan E, Weisz B, Homko CJ, Reece EA, Schiff E. One or two hours postprandial glucose measurements: are they the same? Am J Obstet Gynecol. 2001;185:604–7.
Davidson JA, Stager W, Paranjape S, Berria R, Leiter LA. Achieving postprandial glucose control with lixisenatide improves glycemic control in patients with type 2 diabetes on basal insulin: a post-hoc analysis of pooled data. Clin Diabetes Endocrinol. 2020;6:2.
Hershon KS, Hirsch BR, Odugbesan O. Importance of postprandial glucose in relation to A1C and cardiovascular disease. Clin Diabetes Publ Am Diabetes Assoc. 2019;37:250–9.
Khedkar A, Lebovitz H, Fleming A, Cherrington A, Jose V, Athalye SN, et al. Pharmacokinetics and pharmacodynamics of insulin tregopil in relation to premeal dosing time, between meal interval, and meal composition in patients with type 2 diabetes mellitus. Clin Pharmacol Drug Dev. 2020;9:74–86.
Gregory JM, Lautz M, Moore LM, Williams PE, Reddy P, Cherrington AD. Enterically delivered insulin tregopil exhibits rapid absorption characteristics and a pharmacodynamic effect similar to human insulin in conscious dogs. Diabetes Obes Metab. 2019;21:160–9.
Alqahtani MS, Kazi M, Alsenaidy MA, Ahmad MZ. Advances in oral drug delivery. Front Pharmacol [Internet]. 2021 [cited 2023 Mar 23];12. https://doi.org/10.3389/fphar.2021.618411.
Primavera R, Kevadiya BD, Swaminathan G, Wilson RJ, De Pascale A, Decuzzi P, et al. Emerging nano- and micro-technologies used in the treatment of type-1 diabetes. Nanomaterials. 2020;10:789.
Sonia TA, Sharma CP. 7 - Summary and future perspectives for oral insulin delivery. In: Sonia TA, Sharma CP, editors. Oral Deliv Insul [Internet]. Woodhead Publishing; 2014 [cited 2022 Jun 21]. p. 311–32. https://www.sciencedirect.com/science/article/pii/B9781907568473500070.
Elsayed AM. Oral delivery of insulin: novel approaches | IntechOpen; 2020 [cited 2020 Dec 11]. https://www.intechopen.com/books/recent-advances-in-novel-drug-carrier-systems/oral-delivery-of-insulin-novel-approaches.
Nobex reports oral insulin clinical trial promising. Free Online Library [Internet]; 2020 [cited 2020 Dec 11]. https://www.thefreelibrary.com/NOBEX+REPORTS+ORAL+INSULIN+CLINICAL+TRIAL+PROMISING.-a0114273122.
Kumar BR, Satish SM. Growth strategies of Indian pharma companies. Hyderabad: ICFAI University Press; 2007.
Weiss M, Steiner DF, Philipson LH. Insulin biosynthesis, secretion, structure, and structure-activity relationships. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dungan K, et al., editors. Endotext. South Dartmouth: MDText.com, Inc.; 2000.
Hazra P, Adhikary L, Dave N, Khedkar A, Manjunath HS, Anantharaman R, et al. Development of a process to manufacture PEGylated orally bioavailable insulin. Biotechnol Prog. 2010;26:1695–704.
Clement S, Still JG, Kosutic G, McAllister RG. Oral insulin product hexyl-insulin monoconjugate 2 (HIM2) in type 1 diabetes mellitus: the glucose stabilization effects of HIM2. Diabetes Technol Ther. 2002;4:459–66.
Dave N, Hazra P, Khedkar A, Manjunath HS, Iyer H, Suryanarayanan S. Process and purification for manufacture of a modified insulin intended for oral delivery. J Chromatogr A. 2008;1177:282–6.
PubChem. Insulin tregopil [Internet]; 2020 [cited 2020 Dec 11]. https://pubchem.ncbi.nlm.nih.gov/compound/118984463.
Maher S, Leonard TW, Jacobsen J, Brayden DJ. Safety and efficacy of sodium caprate in promoting oral drug absorption: from in vitro to the clinic. Adv Drug Deliv Rev. 2009;61:1427–49.
Arbit E, Kidron M. Oral insulin: the rationale for this approach and current developments. J Diabetes Sci Technol. 2009;3:562–7.
Tokarz VL, MacDonald PE, Klip A. The cell biology of systemic insulin function. J Cell Biol. 2018;217:2273–89.
Edgerton DS, Scott M, Farmer B, Williams PE, Madsen P, Kjeldsen T, et al. Targeting insulin to the liver corrects defects in glucose metabolism caused by peripheral insulin delivery. JCI Insight. 2019;5: e126974.
Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018;98:2133–223.
van Dijk PR, Logtenberg SJJ, Gans ROB, Bilo HJG, Kleefstra N. Intraperitoneal insulin infusion: treatment option for type 1 diabetes resulting in beneficial endocrine effects beyond glycaemia. Clin Endocrinol (Oxf). 2014;81:488–97.
Guimarães C, Marra CA, Gill S, Meneilly G, Simpson S, Godoy AL, et al. Exploring patients’ perceptions for insulin therapy in type 2 diabetes: a Brazilian and Canadian qualitative study. Patient Prefer Adherence. 2010;4:171–9.
Food and Drug Administration. Guidance for industry: estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. Cent Drug Eval Res CDER. 2005;7.
Khedkar A, Iyer H, Anand A, Verma M, Krishnamurthy S, Savale S, et al. A dose range finding study of novel oral insulin (IN-105) under fed conditions in type 2 diabetes mellitus subjects. Diabetes Obes Metab. 2010;12:659–64.
Heinemann L. Variability of insulin absorption and insulin action. Diabetes Technol Ther. 2002;4:673–82.
Vora J, Heise T. Variability of glucose-lowering effect as a limiting factor in optimizing basal insulin therapy: a review. Diabetes Obes Metab. 2013;15:701–12.
Gin H, Hanaire-Broutin H. Reproducibility and variability in the action of injected insulin. Diabetes Metab. 2005;31:7–13.
Monnier L, Colette C, Dunseath GJ, Owens DR. The loss of postprandial glycemic control precedes stepwise deterioration of fasting with worsening diabetes. Diabetes Care. 2007;30:263–9.
Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care. 2003;26:881–5.
Gerich JE. Clinical significance, pathogenesis, and management of postprandial hyperglycemia. Arch Intern Med. 2003;163:1306–16.
Biocon Limited. An open label, multi-center, randomized, parallel group phase II/III clinical study to evaluate the efficacy and safety of insulin tregopil (IN-105) compared with insulin aspart in the treatment of patients with type 2 diabetes mellitus on stable dose of metformin and insulin glargine [Internet]. clinicaltrials.gov; 2020 May. Report No.: results/NCT03430856. https://clinicaltrials.gov/ct2/show/results/NCT03430856.
Abdul-Ghani MA, Abdul-Ghani T, Ali N, Defronzo RA. One-hour plasma glucose concentration and the metabolic syndrome identify subjects at high risk for future type 2 diabetes. Diabetes Care. 2008;31:1650–5.
Abdul-Ghani MA, Lyssenko V, Tuomi T, DeFronzo RA, Groop L. Fasting versus postload plasma glucose concentration and the risk for future type 2 diabetes: results from the Botnia Study. Diabetes Care. 2009;32:281–6.
Lebovitz HE, Fleming A, Cherrington AD, Joshi S, Athalye SN, Loganathan S, et al. Efficacy and safety of tregopil, a novel, ultra-rapid acting oral prandial insulin analog, as part of a basal-bolus regimen in type 2 diabetes: a randomized, active-controlled phase 2/3 study. Expert Opin Pharmacother. 2022;23:1855–63.
Bowering K, Case C, Harvey J, Reeves M, Sampson M, Strzinek R, et al. Faster aspart versus insulin aspart as part of a basal-bolus regimen in inadequately controlled type 2 diabetes: the onset 2 trial. Diabetes Care. 2017;40:951–7.
Scheen AJ. Clinical efficacy of acarbose in diabetes mellitus: a critical review of controlled trials. Diabetes Metab. 1998;24:311–20.
Guardado-Mendoza R, Prioletta A, Jiménez-Ceja LM, Sosale A, Folli F. The role of nateglinide and repaglinide, derivatives of meglitinide, in the treatment of type 2 diabetes mellitus. Arch Med Sci AMS. 2013;9:936–43.
Choe EY, Cho Y, Choi Y, Yun Y, Wang HJ, Kwon O, et al. The effect of DPP-4 inhibitors on metabolic parameters in patients with type 2 diabetes. Diabetes Metab J. 2014;38:211–9.
Scheen AJ. Reduction in HbA1c with SGLT2 inhibitors vs DPP-4 inhibitors as add-ons to metformin monotherapy according to baseline HbA1c: a systematic review of randomized controlled trials. Diabetes Metab. 2020;46:186–96.
Schrot RJ. Targeting plasma glucose: preprandial versus postprandial. Clin Diabetes. 2004;22:169–72.
Monnier L, Colette C. Target for glycemic control: concentrating on glucose. Diabetes Care. 2009;32:S199-204.
Suryanarayan S, Khedkar A, Vedala A, Iyer H, Anil K, Desai S, et al. Pharmacokinetics and pharmacodynamics of a single oral dose of the insulin analog IN-105 tablet form, in normal healthy volunteers, in the presence of food. Diabetologia. New York: Springer; 2007. p. S95–6.
Khedkar A, Lebovitz H, Fleming A, Cherrington A, Jose V, Athalye SN, et al. Impact of insulin tregopil and its permeation enhancer on pharmacokinetics of metformin in healthy volunteers: randomized, open-label, placebo-controlled, crossover study. Clin Transl Sci. 2019;12:276–82.
Zijlstra E, Heinemann L, Plum-Mörschel L. Oral insulin reloaded: a structured approach. J Diabetes Sci Technol. 2014;8:458–65.
Diabetology Ltd—Projects [Internet] [cited 2022 May 12]. https://www.diabetology.co.uk/projects/.
Luzio SD, Dunseath G, Lockett A, Broke-Smith TP, New RR, Owens DR. The glucose lowering effect of an oral insulin (Capsulin) during an isoglycaemic clamp study in persons with type 2 diabetes. Diabetes Obes Metab. 2010;12:82–7.
Diasome Pharmaceuticals. A single-blind, placebo-controlled, dose-ranging trial of oral HDV-insulin in patients with type 2 diabetes mellitus [Internet]. clinicaltrials.gov; 2021 May. Report No.: NCT00521378. https://clinicaltrials.gov/ct2/show/NCT00521378.
Diasome Pharmaceuticals. An 18-week randomized, double-blind, multicenter, comparator study of two doses of oral HDV-insulin and placebo with background metformin treatment in patients with type 2 diabetes mellitus [Internet]. clinicaltrials.gov; 2021 May. Report No.: NCT00814294. https://clinicaltrials.gov/ct2/show/NCT00814294.
Hirlekar RS. Oral insulin delivery: novel strategies. Asian J Pharm AJP. 2017;11.
Novo Nordisk A/S. A trial investigating the safety, tolerability, pharmacokinetics and pharmacodynamics of NNC 0148-0000-0106 in healthy subjects and subjects with type 1 and type 2 diabetes [Internet]. clinicaltrials.gov; 2017 Jul. Report No.: NCT01028404. https://clinicaltrials.gov/ct2/show/NCT01028404.
Novo Nordisk A/S. A trial investigating the safety, tolerability, pharmacokinetics and pharmacodynamics of NNC 0123-0000-0338 in healthy subjects [Internet]. clinicaltrials.gov; 2017 Feb. Report No.: NCT01334034. https://clinicaltrials.gov/ct2/show/NCT01334034.
Novo Nordisk A/S. A trial investigating the pharmacokinetics and pharmacodynamics of NNC0123-0000-0338 in a tablet formulation with three different coatings in healthy subjects [Internet]. clinicaltrials.gov; 2014 Jan. Report No.: NCT01931137. https://clinicaltrials.gov/ct2/show/NCT01931137.
Novo Nordisk A/S. A multiple dose trial investigating the safety, tolerability, pharmacokinetics and pharmacodynamics of NNC0123-0000-0338 in subjects with type 2 diabetes [Internet]. clinicaltrials.gov; 2017 Feb. Report No.: NCT01796366. https://clinicaltrials.gov/ct2/show/NCT01796366.
Insulin oral (NN 1954)—Novo Nordisk—AdisInsight [Internet] [cited 2022 Jun 21]. https://adisinsight.springer.com/drugs/800036594.
Rachmiel M, Barash G, Leshem A, Sagi R, Doenyas-barak K, Koren S. OR14-1 pharmacodynamics, safety, tolerability, and efficacy of oral insulin formulation (Oshadi Icp) among young adults with type 1 diabetes: a summary of clinical studies phases I, Ib, and II. J Endocr Soc. 2019;3:OR14-1.
Oshadi Drug Administration. A single-center, multiple-dose, randomized, cross-over, double-blind, placebo-controlled study to evaluate the pharmacodynamics, safety, and tolerability of Oshadi Icp in patients with type 1 diabetes mellitus—phase Ib clinical study [Internet]. clinicaltrials.gov; 2013 Oct. Report No.: NCT01772251. https://clinicaltrials.gov/ct2/show/NCT01772251.
Oshadi Drug Administration. A single center, non-randomized, single blind, placebo controlled, single dose study of the safety and efficacy of single administration of Oshadi oral insulin in type I diabetes patients—phase 1 study [Internet]. clinicaltrials.gov; 2012 Apr. Report No.: NCT01120912. https://clinicaltrials.gov/ct2/show/NCT01120912.
The University of Texas Health Science Center at San Antonio. A euglycemic insulin clamp study in type 1 diabetic patients with oral insulin (ORAMED) [Internet]. clinicaltrials.gov; 2019 Mar. Report No.: results/NCT02535715. https://clinicaltrials.gov/ct2/show/results/NCT02535715.
Hadassah Medical Organization. A single blind, open-label study to assess the safety, pharmacokinetics and pharmacodynamics of oral insulin formulation in type 1 subjects [Internet]. clinicaltrials.gov; 2011 Aug. Report No.: NCT00867594. https://clinicaltrials.gov/ct2/show/NCT00867594.
Oramed, Ltd. Randomized, double-blind, placebo-controlled study to assess the safety, PK and PD of multiple oral bedtime doses of ORMD-0801 in adult patients with T2DM who are inadequately controlled with diet and exercise or diet, exercise and metformin [Internet]. clinicaltrials.gov; 2015 Mar. Report No.: NCT01889667. https://clinicaltrials.gov/ct2/show/NCT01889667.
Acknowledgements
The authors acknowledge Ashwini Vishweswaramurthy for her contributions towards the development of this monograph, Shivani Mittra and Geetanjali Tonpe for writing support and Jayanti Panda for operational support. Authors also acknowledge Molecular Connections Analytics Pvt. Ltd., Bengaluru, for providing editorial support towards its development.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Shashank Joshi has received Speaker/Advisory/Research Grants from Abbott, Alkem, Astra Zeneca, Bayer, Biocon, Boehringer Ingelheim, Eli Lilly, Franco Indian, Glenmark, Lupin, Marico, MSD, Novartis, Novo Nordisk, Roche, Sanofi, Serdia, Torrent Pharma, Twin Health and Zydus. Sandeep N. Athalye, Subramanian Loganathan, and Vathsala Jayanth are currently employed with Biocon Biologics Ltd and Vasan K. Sambandamurthy is previous employee of Biocon Limited.
Funding
Biocon Limited.
Conflict of Interest
S.N.A, S.L, V.J, and V.K.S. hold stocks in Biocon. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest or financial conflict with the subject matter or materials discussed in the review apart from those disclosed. For S.J., please refer to ‘Disclosures’.
Data Availability
The data supporting the study findings are available from the corresponding author, Subramanian Loganathan, upon reasonable request.
Ethics Approval
The ethical standards of independent ethics committees or institutional review board were followed during the conduct of the clinical trials included in this review.
Consent to Participate
All the subjects involved in the clinical trials mentioned in this review received informed consent prior to the study-related procedures, which was duly signed by the subjects and the investigators.
Consent for Publication
Not applicable.
Code Availability
Not applicable.
Author Contributions
S.J., V.J., S.L., V.K.S., and S.N.A. designed and conceptualized the review article. All the authors were involved in writing, reviewing and approving the manuscript. As the guarantor of this work, S.L., takes full responsibility for the work, including the decision to submit and publish the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Joshi, S., Jayanth, V., Loganathan, S. et al. Insulin Tregopil: An Ultra-Fast Oral Recombinant Human Insulin Analog: Preclinical and Clinical Development in Diabetes Mellitus. Drugs 83, 1161–1178 (2023). https://doi.org/10.1007/s40265-023-01925-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-023-01925-1