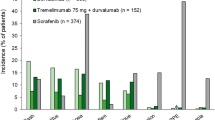
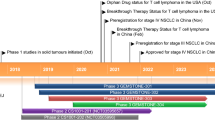
Abstract
Tremelimumab (tremelimumab-actl; IMJUDO®), a cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) blocking antibody, is being developed by AstraZeneca, under license from Pfizer, for the treatment of a range of malignant tumours. Tremelimumab was approved in the USA in October 2022 in combination with durvalumab for the treatment of adult patients with unresectable hepatocellular carcinoma (uHCC). In addition, tremelimumab in combination with durvalumab and platinum-based chemotherapy was approved in the USA in November 2022 for the treatment of adult patients with metastatic non-small cell lung cancer (mNSCLC) with no sensitizing epidermal growth factor receptor mutation or anaplastic lymphoma kinase genomic tumour aberrations. In December 2022, tremelimumab in combination with durvalumab received a Positive Opinion from the EU Committee for Medicinal Products for Human Use for the first line treatment of adults with advanced or unresectable HCC. Tremelimumab in combination with durvalumab is under regulatory review for these indications in Japan and in other countries worldwide. This article summarizes the milestones in the development of tremelimumab leading to this first approval.
Similar content being viewed by others
References
Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8(9):1069–86.
AstraZeneca. Tremelimumab (IMJUDO®; tremelimumab-actl): US prescribing information. 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761270s000lbl.pdf. Accessed 14 Nov 2022.
US Food & Drug Administration. FDA approves tremelimumab in combination with durvalumab for unresectable hepatocellular carcinoma [media release]. 24 October 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tremelimumab-combination-durvalumab-unresectable-hepatocellular-carcinoma.
AstraZeneca. Imjudo (tremelimumab) in combination with Imfinzi approved in the US for patients with unresectable liver cancer [media release]. 24 October 2022. https://www.astrazeneca.com/.
US Food & Drug Administration. FDA approves tremelimumab in combination with durvalumab and platinum-based chemotherapy for metastatic non-small cell lung cancer [media release]. 10 Nov 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tremelimumab-combination-durvalumab-and-platinum-based-chemotherapy-metastatic-non.
AstraZeneca. Imfinzi and Imjudo with chemotherapy approved in the US for patients with metastatic non-small cell lung cancer [media release]. 11 Nov 2022. https://www.astrazeneca.com/.
European Medicines Agency. Imjudo (tremelimumab): summary of opinion. 2022. https://www.ema.europa.eu/en/medicines/human/summaries-opinion/imjudo. Accessed 19 Dec 2022.
Lilly. Lilly and AstraZeneca expand immuno-oncology research collaboration with new combinations [media release]. 22 Oct 2015. https://www.lilly.com/.
AstraZeneca. Medimmune and Immunocore announce new collaboration to conduct immuno-oncology combination trials in melanoma [media release]. 16 Apr 2015. https://www.astrazeneca.com/.
Kyowa Hakko Kirin Co Ltd. Kyowa Hakko Kirin and AstraZeneca partner on immuno-oncology clinical study [media release]. 30 Jul 2014. http://www.kyowa-kirin.com.
MedImmune. MedImmune joins forces with leading cancer organizations to advance novel immunotherapy research [media release]. 9 Oct 2012. https://www.astrazeneca.com/.
MedImmune. MedImmune in-licenses cancer immunotherapy tremelimumab from Pfizer [media release]. 3 Oct 2011. https://www.astrazeneca.com.
Hanson DC, Canniff PC, Primiano MJ, et al. Preclinical in vitro characterization of anti-CTLA4 therapeutic antibody CP-675,206 [abstract no 3802]. Cancer Res. 2004;64(7 Suppl):877.
Cheng L, Creasy T, Pilataxi F, et al. Effects of combination treatment with durvalumab plus tremelimumab on the tumor microenvironment in non-small-cell lung carcinoma. Cancer Immunol Immunother. 2022;71(5):1167–81.
Kelley RK, Sangro B, Harris W, et al. Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: randomized expansion of a phase I/II study. J Clin Oncol. 2021;39(27):2991–3001.
McCoon P, Lee YS, Kelley RK, et al. T cell receptor pharmacodynamics associated with survival and response to tremelimumab (T) in combination with durvalumab (D) in patients (pts) with unresectable hepatocellular carcinoma (uHCC) [abstract no 4087]. J Clin Oncol. 2021;39(15 Suppl):4087.
Abou-Alfa GK, Lau G, Kudo M, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evidence. 2022. https://doi.org/10.1056/EVIDoa2100070.
Johnson ML, Cho BC, Luft A, et al. Durvalumab with or without tremelimumab in combination with chemotherapy as first-line therapy for metastatic non-small-cell lung cancer: the phase III POSEIDON study. J Clin Oncol. 2022. https://doi.org/10.1200/JCO.22.00975.
Johnson M, Cho BC, Luft A, et al. Durvalumab (D) ± tremelimumab (T) + chemotherapy (CT) in 1L metastatic (m) NSCLC: overall survival (OS) update from POSEIDON after median follow-up (mFU) of approximately 4 years (y) [abstract no LBA59]. Ann Oncol J. 2022;33(Suppl 7):S1424–5.
de Castro G, Jr., Rizvi NA, Schmid P, et al. NEPTUNE: phase III study of first-line durvalumab plus tremelimumab in patients with metastatic NSCLC. J Thorac Oncol. 2022. https://doi.org/10.1016/j.jtho.2022.09.223.
Rizvi NA, Cho BC, Reinmuth N, et al. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer: the MYSTIC phase 3 randomized clinical trial. JAMA Oncol. 2020;6(5):661–74.
Planchard D, Reinmuth N, Orlov S, et al. ARCTIC: durvalumab with or without tremelimumab as third-line or later treatment of metastatic non-small-cell lung cancer. Ann Oncol. 2020;31(5):609–18.
Goldman JW, Dvorkin M, Chen Y, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):51–65.
Paz-Ares L, Chen Y, Reinmuth N, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year overall survival update from CASPIAN. ESMO Open. 2022;7(2): 100408.
Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum–etoposide versus platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. The Lancet. 2019;394(10212):1929–39.
Powles T, van der Heijden MS, Castellano D, et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020;21(12):1574–88.
Cuellar MA, Medina A, Girones R, et al. Phase II trial of durvalumab plus tremelimumab with concurrent radiotherapy as bladder-sparing therapy in patients with localized muscle invasive bladder cancer: a SOGUG study [abstract no. TPS5097]. J Clin Oncol. 2020;38(15 Suppl).
del Muro XG, Valderrama BP, Medina A, et al. Phase II trial of durvalumab plus tremelimumab with concurrent radiotherapy (RT) in patients (pts) with localized muscle invasive bladder cancer (MIBC) treated with a selective bladder preservation approach: IMMUNOPRESERVE-SOGUG trial [abstract no. 4505]. J Clin Oncol. 2021;39(15 Suppl).
Grande E, Guerrero F, Puente J, et al. DUTRENEO trial: a randomized phase II trial of DUrvalumab and TREmelimumab versus chemotherapy as a NEOadjuvant approach to muscle-invasive urothelial bladder cancer (MIBC) patients (pts) prospectively selected by an interferon (INF)-gamma immune signature [abstract no. 5012]. J Clin Oncol. 2020;38(15 Suppl).
Delaye M, Assenat E, Dahan L, et al. Durvalumab (D) plus tremelimumab (T) immunotherapy in patients (Pts) with advanced biliary tract carcinoma (BTC) after failure of platinum-based chemotherapy (CTx): interim results of the IMMUNOBIL GERCOR D18-1 PRODIGE-57 study [abstract no. 4108]. J Clin Oncol. 2022;40(16 Suppl).
Castillon CJ, Plana M, Castelo B, et al. Durvalumab (D) plus tremelimumab (T) for the treatment of patients with progressive, refractory advanced thyroid carcinoma: the DUTHY (GETNE-T1812) trial [abstract no 1645O]. Ann Oncol. 2022;33(Suppl 7):S1294–5.
Frenel JS, Hervieu A, Borcoman E, et al. Tremelimumab (T) + durvalumab (D) combined with metronomic oral vinorelbine (MOV): results of the recurrent cervical cancer (RCC) cohort of the MOVIE study [abstract no 775P]. Ann Oncol. 2021;32(Suppl 5):S753.
Hervieu A, Guigay J, Borcoman E, et al. Metronomic oral vinorelbine (MOV) combined with tremelimumab (T) + durvalumab (D): results of the tumor mutational burden-high (TMB-h) and/or microsatellite instability-high (MSI-h) cohort of the MOVIE study [abstract no 478P]. Ann Oncol. 2022;33(Suppl 7):S758–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Susan J. Keam is a contracted employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethical approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Keam, S.J. Tremelimumab: First Approval. Drugs 83, 93–102 (2023). https://doi.org/10.1007/s40265-022-01827-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-022-01827-8