Abstract
Background
Polycystic ovary syndrome (PCOS) is the most common endocrine condition in women, impacting several aspects of a woman's life, including reproductive, mental, cardiovascular, and metabolic health. Antidiabetic drugs may have beneficial effects on the endocrine and metabolic states in women with PCOS.
Objective
This study aimed to compare the effects of oral antidiabetic drugs on reproductive hormones, metabolic and anthropometric markers, and menstrual frequency, in patients with PCOS using network meta-analysis.
Methods
PubMed, EMBASE, and CENTRAL were searched for studies published up to May 31, 2021. Randomised clinical trials enrolling participants with PCOS were included, for which sodium-glucose cotransporter 2 (SGLT-2) inhibitors, metformin (Met), dipeptidyl peptidase 4 (DPP-4) inhibitors, alpha-glucosidase inhibitors, glucagon-like peptide 1 receptor agonists (GLP-1 RAs), and thiazolidinediones (TZDs) (alone or in combination) were compared with either each other, placebo, or no treatment. A network meta-analysis using a Bayesian approach was performed. The outcomes included changes in endocrine outcomes, metabolic results, menstrual frequency, and anthropometric findings. All research was conducted according to a protocol registered in the PROSPERO database (CRD42021248314).
Results
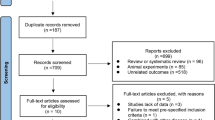
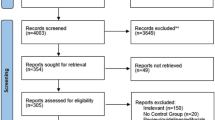
In total, we retrieved 3383 studies, of which 47 articles enrolling 2626 participants were included for the network meta-analysis. In comparison to the control groups, Met (MD − 0.41, 95% CI − 0.73 to − 0.09) was more beneficial in reducing serum total testosterone, and GLP-1RAs+Met (MD − 5.44, 95% CI − 10.06 to − 0.89) reduced free androgen index (FAI) more effectively. Thiazolidinediones (MD 9.33, 95% CI 0.15 to 17.99) had a greater effect on sex hormone-binding globulin (SHBG) than Met. For decreasing androstenedione, Met (MD − 1.87, 95% CI − 2.73 to − 1.01), DPP-4 inhibitors (MD − 2.64, 95% CI − 4.77 to − 0.49), GLP-1RAs (MD − 3.06, 95% CI − 5.53 to − 0.62), and GLP-1RAs+Met (MD − 2.97, 95% CI − 5.85 to − 0.09) were more effective than TZDs. The confidence in evidence was often low or very low.
Conclusions
In this network meta-analysis, GLP-1 receptor agonists in combination with metformin appear to be preferable for improving hyperandrogenaemia. Metformin and TZDs offer the added benefit of improving fasting blood glucose (FBG) and low-density lipoprotein-cholesterol (LDL-C) when compared to the control groups. Metformin combined with GLP-1 receptor agonists or TZDs could be associated with a beneficial effect on menstrual recovery.

Similar content being viewed by others
References
Meier RK. Polycystic ovary syndrome. Nurs Clin North Am. 2018;53(3):407–20.
Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38(9):1165–74.
Papaetis GS, Filippou PK, Constantinidou KG, Stylianou CS. Liraglutide: new perspectives for the treatment of polycystic ovary syndrome. Clin Drug Investig. 2020;40(8):695–713.
Toosy S, Sodi R, Pappachan JM. Lean polycystic ovary syndrome (PCOS): an evidence-based practical approach. J Diabetes Metab Disord. 2018;17(2):277–85.
Street ME, Cirillo F, Catellani C, Dauriz M, Lazzeroni P, Sartori C, et al. current treatment for polycystic ovary syndrome: focus on adolescence. Minerva Pediatr. 2020;72(4):288–311.
Morley LC, Tang T, Yasmin E, Norman RJ, Balen AH. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, d-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst Rev. 2017;11(11):Cd003053.
Li Y, Tan J, Wang Q, Duan C, Hu Y, Huang W. Comparing the individual effects of metformin and rosiglitazone and their combination in obese women with polycystic ovary syndrome: a randomized controlled trial. Fertil Steril. 2020;113(1):197–204.
Javed Z, Papageorgiou M, Madden LA, Rigby AS, Kilpatrick ES, Atkin SL, et al. The effects of empagliflozin vs metformin on endothelial microparticles in overweight/obese women with polycystic ovary syndrome. Endocr Connect. 2020;9(6):563–9.
Ferjan S, Janez A, Jensterle M. DPP4 inhibitor sitagliptin as a potential treatment option in metformin-intolerant obese women with polycystic ovary syndrome: a pilot randomized study. Endocr Pract. 2018;24(1):69–77.
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210.
Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25.
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:Ed000142.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343: d5928.
Huhn M, Nikolakopoulou A, Schneider-Thoma J, Krause M, Samara M, Peter N, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939–51.
Nikolakopoulou A, Higgins JPT, Papakonstantinou T, Chaimani A, Del Giovane C, Egger M, et al. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020;17(4): e1003082.
Furukawa TA, Salanti G, Atkinson LZ, Leucht S, Ruhe HG, Turner EH, et al. Comparative efficacy and acceptability of first-generation and second-generation antidepressants in the acute treatment of major depression: protocol for a network meta-analysis. BMJ Open. 2016;6(7): e010919.
Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. 2012;3(2):98–110.
Han Y, Li Y, He B. GLP-1 receptor agonists versus metformin in PCOS: a systematic review and meta-analysis. Reprod Biomed Online. 2019;39(2):332–42.
Xing C, Li C, He B. Insulin sensitizers for improving the endocrine and metabolic profile in overweight women with PCOS. J Clin Endocrinol Metab. 2020;105(9):2950–63.
Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E. American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and Pcos Society Disease State clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome-part 1. Endocr Pract. 2015;21(11):1291–300.
Elkind-Hirsch K, Marrioneaux O, Bhushan M, Vernor D, Bhushan R. Comparison of single and combined treatment with exenatide and metformin on menstrual cyclicity in overweight women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(7):2670–8.
Hanjalic-Beck A, Gabriel B, Schaefer W, Zahradnik HP, Schories M, Tempfer C, et al. Metformin versus acarbose therapy in patients with polycystic ovary syndrome (PCOS): a prospective randomised double-blind study. Gynecol Endocrinol. 2010;26(9):690–7.
Javed Z, Papageorgiou M, Deshmukh H, Rigby AS, Qamar U, Abbas J, et al. Effects of empagliflozin on metabolic parameters in polycystic ovary syndrome: a randomized controlled study. Clin Endocrinol (Oxf). 2019;90(6):805–13.
Seufert J, Laubner K. Outcome studies on SGLT-2 inhibitors. Internist (Berl). 2019;60(9):903–11.
Zheng SL, Roddick AJ, Aghar-Jaffar R, Shun-Shin MJ, Francis D, Oliver N, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319(15):1580–91.
Fitchett D, Inzucchi SE, Cannon CP, McGuire DK, Scirica BM, Johansen OE, et al. Empagliflozin reduced mortality and hospitalization for heart failure across the spectrum of cardiovascular risk in the EMPA-REG OUTCOME trial. Circulation. 2019;139(11):1384–95.
Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776–85.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None.
Conflict of interest
SY, YCM, WH and LZ declare no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Appendix 15 in the Supplementary Information.
Author contributions
SY designed the study. YCM and WH identified and selected trials and extracted data. SY and LZ performed all the data analysis, checked for statistical inconsistency, and interpreted the data. SY contributed to data interpretation. SY drafted the paper, and all other authors critically reviewed the paper. All authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, S., Zhao, L., He, W. et al. The Effect of Oral Antidiabetic Drugs on Improving the Endocrine and Metabolic States in Women with Polycystic Ovary Syndrome: A Systematic Review and Network Meta-analysis. Drugs 82, 1469–1480 (2022). https://doi.org/10.1007/s40265-022-01779-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-022-01779-z