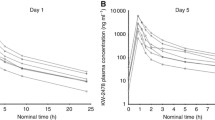
Abstract
Pimitespib (Jeselhy®) is an oral small molecule inhibitor of the α and β isoforms of heat shock protein 90 (HSP90). HSP90α and HSP90β regulate the stability and activity of a number of proteins that are crucial for tumour development. Pimitespib is being developed by Taiho Pharmaceutical for the treatment of solid tumours, including gastrointestinal stromal tumour (GIST), and in June 2022 it received its first approval in Japan for GIST that has progressed after chemotherapy. Pimitespib is undergoing phase I development for the treatment of solid tumours in the EU and the USA. This article summarizes the milestones in the development of pimitespib leading to this first approval for GIST that has progressed after chemotherapy.
Similar content being viewed by others
References
Søreide K, Sandvik OM, Søreide JA, et al. Global epidemiology of gastrointestinal stromal tumours (GIST): a systematic review of population-based cohort studies. Cancer Epidemiol. 2016;40:39–46.
US Library of Medicine. Genetics Home Reference: gastrointestinal stromal tumor. 2021. https://medlineplus.gov/genetics/condition/gastrointestinal-stromal-tumor/. Accessed 15 Jul 2022.
Kelly CM, Gutierrez Sainz L, Chi P. The management of metastatic GIST: current standard and investigational therapeutics. J Hematol Oncol. 2021;14(1):2.
Napolitano A, Vincenzi B. Secondary KIT mutations: the GIST of drug resistance and sensitivity. Br J Cancer. 2019;120(6):577–8.
Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475(7356):324–32.
Yoshimura C, Nagatoishi S, Kuroda D, et al. Thermodynamic dissection of potency and selectivity of cytosolic HSP90 inhibitors. J Med Chem. 2021;64(5):2669–77.
Kim K, Lee HW, Lee EH, et al. Differential expression of HSP90 isoforms and their correlations with clinicopathologic factors in patients with colorectal cancer. Int J Clin Exp Pathol. 2019;12(3):978–86.
Ohkubo S, Kodama Y, Muraoka H, et al. TAS-116, a highly selective inhibitor of heat shock protein 90 alpha and beta, demonstrates potent antitumor activity and minimal ocular toxicity in preclinical models. Mol Cancer Ther. 2015;14(1):14–22.
Taiho Pharmaceutical Co. Ltd. Taiho Pharmaceutical obtains approval to manufacture and market HSP90 inhibitor Jeselhy® tablets 40 mg (pimitespib) for gastrointestinal stromal tumor (GIST) [media release]. 20 June 2022. https://www.taiho.co.jp/en/release/2022/20220620.html.
Taiho Pharmaceutical Co. Ltd. Jeselhy® (pimitespib) tablets: Japanese prescribing information; 2022. https://www.info.pmda.go.jp/go/pack/42910F6F1029_1_01/. Accessed 27 June 2022.
Taiho Pharmaceutical Co. Ltd. Product pipeline; 2022. https://www.taiho.co.jp/en/science/pipeline/. Accessed 18 Jul 2022.
Saito Y, Takahashi T, Obata Y, et al. TAS-116 inhibits oncogenic KIT signalling on the Golgi in both imatinib-naive and imatinib-resistant gastrointestinal stromal tumours. Br J Cancer. 2020;122(5):658–67.
Shimomura A, Yamamoto N, Kondo S, et al. First-in-human phase I study of an oral HSP90 inhibitor, TAS-116, in patients with advanced solid tumors. Mol Cancer Ther. 2019;18(3):531–40.
Kurokawa Y, Honma Y, Sawaki A, et al. Pimitespib in patients with advanced gastrointestinal stromal tumor (CHAPTER-GIST-301): a randomized, double-blind, placebo-controlled phase 3 trial. Ann Oncol. 2022;7(10):518.
Doi T, Kurokawa Y, Sawaki A, et al. Efficacy and safety of TAS-116, an oral inhibitor of heat shock protein 90, in patients with metastatic or unresectable gastrointestinal stromal tumour refractory to imatinib, sunitinib and regorafenib: a phase II, single-arm trial. Eur J Cancer. 2019;121:29–39.
Kawazoe A, Itahashi K, Yamamoto N, et al. TAS-116 (pimitespib), an oral HSP90 inhibitor, in combination with nivolumab in patients with colorectal cancer and other solid tumors: an open-label, dose-finding, and expansion phase Ib trial (EPOC1704). Clin Cancer Res. 2021;27(24):6709–15.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Sheridan M. Hoy is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hoy, S.M. Pimitespib: First Approval. Drugs 82, 1413–1418 (2022). https://doi.org/10.1007/s40265-022-01764-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-022-01764-6