Abstract
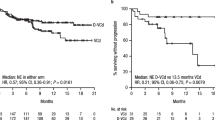
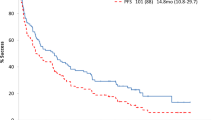
Subcutaneous daratumumab (DARZALEX®) co-formulated with recombinant human hyaluronidase (DARZALEX FASPRO®) is approved in several countries, including the USA and those of the EU, for use in combination with bortezomib, cyclophosphamide and dexamethasone for the treatment of adult patients with newly diagnosed light chain (AL) amyloidosis. Daratumumab is a CD38-targeting, human IgG1κ monoclonal antibody. In the pivotal phase III ANDROMEDA trial in adults with newly diagnosed systemic AL amyloidosis, the addition of daratumumab to bortezomib, cyclophosphamide and dexamethasone significantly increased the proportion of patients achieving a haematological complete response relative to bortezomib, cyclophosphamide and dexamethasone alone (primary endpoint). Daratumumab combination therapy produced rapid and deep haematological responses which were associated with improved major organ deterioration progression-free survival (PFS). The addition of daratumumab also led to higher cardiac and renal response rates at 6 and 12 months. Daratumumab had an acceptable tolerability profile when used as combination therapy. Therefore, daratumumab in combination with bortezomib, cyclophosphamide and dexamethasone represents an important emerging first-line treatment option for patients with systemic AL amyloidosis.
Plain Language Summary
Systemic AL amyloidosis is a rare protein misfolding disease that causes serious damage to different organs, especially the heart and kidneys. Daratumumab (DARZALEX®) is a human monoclonal antibody that targets CD38, a protein expressed on clonal plasma cells. A subcutaneous formulation of daratumumab, co-formulated with recombinant human hyaluronidase (DARZALEX FASPRO®), is approved for use in adult patients with newly diagnosed AL amyloidosis. When used in combination with bortezomib, cyclophosphamide and dexamethasone, daratumumab was associated with higher rates of haematological complete response and prolongation of major organ deterioration PFS compared with bortezomib, cyclophosphamide and dexamethasone alone. The addition of daratumumab was also associated with near doubling of cardiac and renal response rates at 6 and 12 months. Subcutaneous daratumumab had an acceptable tolerability profile when used as combination therapy, with no new safety concerns. The combination of daratumumab with bortezomib, cyclophosphamide and dexamethasone is an important emerging treatment option for patients with newly diagnosed systemic AL amyloidosis.


Similar content being viewed by others
References
Merlini G, Dispenzieri A, Sanchorawala V, et al. Systemic immunoglobulin light chain amyloidosis. Nat Rev Dis Primers. 2018;4(1):38.
Roccatello D, Fenoglio R, Sciascia S, et al. CD38 and anti-CD38 monoclonal antibodies in AL amyloidosis: targeting plasma cells and beyond. Int J Mol Sci. 2020;21(11):4129.
Gertz MA. Immunoglobulin light chain amyloidosis: 2020 update on diagnosis, prognosis, and treatment. Am J Hematol. 2020;95(7):848–60.
Palladini G, Milani P, Merlini G. Management of AL amyloidosis in 2020. Blood. 2020;136(23):2620–7.
Janssen Biotech. DARZALEX FASPRO® (daratumumab and hyaluronidase-fihj) injection, for subcutaneous use: US prescribing information. 2021. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/DARZALEX+Faspro-pi.pdf. Accessed 15 Mar 2022
European Medicines Agency. DARZALEX 800mg solution for injection: EU summary of product characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/darzalex-epar-product-information_en.pdf. Accessed 15 Mar 2022
Blair HA. Daratumumab: a review in relapsed and/or refractory multiple myeloma. Drugs. 2017;77(18):2013–24.
McKeage K. Daratumumab: first global approval. Drugs. 2016;76(2):275–81.
Syed YY. Daratumumab: a review in combination therapy for transplant-ineligible newly diagnosed multiple myeloma. Drugs. 2019;79(4):447–54.
Lamb YN. Daratumumab: a review in combination therapy for transplant-eligible newly diagnosed multiple myeloma. Drugs. 2020;80(14):1455–64.
Luo MM, Zhu PP, Nnane I, et al. Population pharmacokinetics and exposure-response modeling of daratumumab subcutaneous administration in patients with light-chain amyloidosis. J Clin Pharmacol. 2021. https://doi.org/10.1002/jcph.1994.
Kastritis E, Palladini G, Minnema MC, et al. Daratumumab-based treatment for immunoglobulin light-chain amyloidosis. N Engl J Med. 2021;385(1):46–58.
Minnema MC, Dispenzieri A, Merlini G, et al. Outcomes by cardiac stage in newly diagnosed AL amyloidosis: results from Andromeda [abstract]. Blood. 2020;136(Suppl. 1):44–5.
Kumar S, Dispenzieri A, Bhutani D, et al. Evaluating the impact of cytogenetic abnormalities on treatment outcomes in patients with AL amyloidosis: subanalyses from the ANDROMEDA study [abstract no. OAB-034]. In: 18th International Myeloma Workshop. 2021
Suzuki K, Wechalekar AD, Kim K, et al. Subcutaneous daratumumab (DARA SC) + bortezomib, cyclophosphamide, and dexamethasone (VCd) in Asian patients with newly diagnosed light chain (AL) amyloidosis: subgroup analysis from the phase 3 Andromeda study [abstract]. Blood. 2020;136(Suppl. 1):11.
Wechalekar AD, Palladini G, Merlini G, et al. Rapid and deep hematologic responses are associated with improved major organ deterioration progression-free survival in newly diagnosed AL amyloidosis: results from Andromeda [abstract]. Blood. 2020;136(Suppl. 1):6–7.
Comenzo RL, Kastritis E, Palladini G, et al. Reduction in absolute involved free light chain and difference between involved and uninvolved free light chain is associated with prolonged major organ deterioration progression-free survival in patients with newly diagnosed AL amyloidosis receiving bortezomib, cyclophosphamide, and dexamethasone with or without daratumumab: results from ANDROMEDA [abstract]. Blood. 2020;136(Suppl. 1):48–50.
Sanchorawala V, Palladini G, Minnema MC, et al. Health-related quality of life in patients with AL amyloidosis treated with daratumumab, bortezomib, cyclophosphamide, and dexamethasone: results from the phase 3 Andromeda study [abstract]. Blood. 2020;136(Suppl. 1):37–40.
Grogan M, Maurer MS, Witteles R, et al. Effect of daratumumab, bortezomib, cyclophosphamide, and dexamethasone on cardiac function and health-related quality of life in patients with newly-diagnosed AL amyloidosis with cardiac involvement: results from the phase 3 ANDROMEDA study [abstract]. J Am Coll Cardiol. 2021;77(18 Suppl. 1):3304.
Havasi A, Lachmann HJ, Leung N, et al. Effect of daratumumab/bortezomib/cyclophosphamide/dexamethasone on renal function and hrqol in patients with newly-diagnosed AL amyloidosis with renal involvement: results from the phase 3 ANDROMEDA study [abstract no. POS-799]. Kidney Int Rep. 2021;6(4 Suppl.):S347.
Comenzo R, Palladini G, Kastritis E, et al. Subcutaneous daratumumab with bortezomib, cyclophosphamide, and dexamethasone in patients with newly diagnosed light chain (AL) amyloidosis: 18-month analysis of the phase 3 ANDROMEDA study [abstract no. 653]. Blood. 2021;138(Suppl. 1):159.
Palladini G, Kastritis E, Maurer MS, et al. Daratumumab plus CyBorD for patients with newly diagnosed AL amyloidosis: safety run-in results of ANDROMEDA. Blood. 2020;136(1):71–80.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NNCN Guidelines®): systemic light chain amyloidosis (version 1.2022). 2022. http://www.nccn.org. Accessed 15 Mar 2022
Mayo Clinic. mSMART Mayo Consensus on AL amyloidosis: diagnosis, treatment and prognosis. 2020. https://www.msmart.org. Accessed 15 Mar 2022
Palladini G, Schonland SO, Sanchorawala V, et al. Clarification on the definition of complete haematologic response in light-chain (AL) amyloidosis. Amyloid. 2021;28(1):1–2.
Acknowledgements
During the peer review process, the manufacturer of daratumumab was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
Hannah Blair is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
The manuscript was reviewed by: M. Beksac, Department of Hematology, Ankara University, Ankara, Turkey; M. A. Dimopoulos, Department of Clinical Therapeutics, National & Kapodistrian University of Athens, Athens, Greece; G. Palladini, Amyloidosis Research and Treatment Center, Fondazione IRCCS Policlinico San Matteo and Department of Molecular Medicine, University of Pavia, Pavia, Italy.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Blair, H.A. Daratumumab: A Review in Newly Diagnosed Systemic Light Chain Amyloidosis. Drugs 82, 683–690 (2022). https://doi.org/10.1007/s40265-022-01705-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-022-01705-3