Abstract
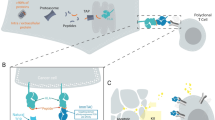
Tebentafusp (tebentafusp-tebn; Kimmtrak®) is a first-in-class, bispecific gp100 peptide-HLA-A*02:01 directed T cell receptor (TCR) CD3 T cell engager being developed by Immunocore for the treatment of uveal melanoma and malignant melanoma. The TCR arm of tebentafusp binds to HLA-A*02:01-positive uveal melanoma cells and activates polyclonal T cells, through CD3, to release inflammatory cytokines and cytolytic proteins, resulting in the direct lysis of tumour cells. In January 2022, tebentafusp received its first approval in the USA for the treatment of HLA-A*02:01-positive adults with unresectable or metastatic uveal melanoma, and in February 2022 received a Positive Opinion from the EU Committee for Medicinal Products for Human Use for the treatment of uveal melanoma. Tebentafusp is under regulatory review for the treatment of metastatic uveal melanoma in the UK, Australia and Canada. Clinical studies of tebentafusp are underway for uveal melanoma and cutaneous melanoma in several countries worldwide. This article summarizes the milestones in the development of tebentafusp leading to this first approval for unresectable or metastatic uveal melanoma.
Similar content being viewed by others
References
Chacon M, Pfluger Y, Angel M, et al. Uncommon subtypes of malignant melanomas: a review based on clinical and molecular perspectives. Cancers. 2020;12(9):1–32.
Martinez-Perez D, Viñal D, Solares I, et al. Gp-100 as a novel therapeutic target in uveal melanoma. Cancers. 2021;13(23):5968.
Damato BE, Dukes J, Goodall H, et al. Tebentafusp: T cell redirection for the treatment of metastatic uveal melanoma. Cancers. 2019;11(7):971.
US Food & Drug Administration. FDA approves tebentafusp-tebn for unresectable or metastatic uveal melanoma [media release]. 25 Jan 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tebentafusp-tebn-unresectable-or-metastatic-uveal-melanoma.
Immunocore. KIMMTRAK® (tebentafusp-tebn): US prescribing information. 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761228s000lbl.pdf. Accessed 10 Feb 2022.
European Medicines Agency. Kimmtrak (tebentafusp): summary of opinion. 2022. https://www.ema.europa.eu/en/medicines/human/summaries-opinion/kimmtrak. Accessed 16 Mar 2022.
AstraZeneca. Medimmune and Immunocore announce new collaboration to conduct immuno-oncology combination trials in melanoma [media release]. 16 Apr 2015. https://www.astrazeneca.com/media-centre/press-releases/2015/medimmune-immunocore-immuno-oncology-combination-trials-melanoma-16042015.html.
CMC Biologics. CMC Biologics announces agreement with Immunocore for process transfer, scale-up and commercial-scale manufacturing of IMCgp100 [media release]. 23 Oct 2021. http://www.cmcbiologics.com.
Immunocore. Immunocore and Medison Pharma partner for future commercialization of tebentafusp in Canada, Central Eastern Europe, and Israel [media release]. 18 Oct 2015. https://ir.immunocore.com/news-releases/news-release-details/immunocore-and-medison-pharma-partner-future-commercialization.
Boudousquie C, Bossi G, Hurst JM, et al. Polyfunctional response by ImmTAC (IMCgp100) redirected CD8(+) and CD4(+) T cells. Immunology. 2017;152(3):425–38.
Gascoyne D, Petrovic K, Ranade K, et al. IL-2 augments immtac-dependent T cell activation and tumour cell killing [abstract no. 628]. J Immunother Cancer. 2020;8(Suppl 3):A377.
Butler MO, Stanhope S, Naidoo R, et al. Tebentafusp induces transient systemic inflammation and modifies the micro-environment to sensitize uveal melanoma tumors to cytotoxic CD8 cells [abstract no. 517]. Cancer Research Conference: AACR Annual Meeting. 2021;81(13 Suppl).
Middleton MR, Steven NM, Evans TJ, et al. Pharmacodynamic effect of tebentafusp (TCR-CD3 bispecific) on peripheral cytokines and association with OS in patients with advanced melanoma [abstract]. Pigment Cell Melanoma Res. 2020;33(1):231.
Stager R, Stanhope S, Greenshields-Watson A, et al. Demonstration of T cell redirection and immune activation in skin rash following tebentafusp treatment [abstract no. 1772P]. Ann Oncol. 2021;32(Suppl 5):S1215.
Vardeu M, Depoil D, Britton-Rivet C, et al. IFNg secreted by tebentafusp (IMCgp100)-redirected t cells inhibits expression of melanin synthesis pathway genes in healthy melanocytes [abstract no. 624]. J Immunother Cancer. 2020;8(Suppl 3):A375.
Middleton MR, Steven NM, Evans TJ, et al. Safety, pharmacokinetics and efficacy of IMCgp100, a first-in-class soluble TCR-antiCD3 bispecific t cell redirector with solid tumour activity: results from the FIH study in melanoma [abstract]. J Clin Oncol. 2016;34(15 suppl):3016.
Nathan P, Hassel JC, Rutkowski P, et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N Engl J Med. 2021;385(13):1196–206.
Sato T, Nathan PD, Hernandez-Aya L, et al. Redirected T cell lysis in patients with metastatic uveal melanoma with gp100-directed TCR IMCgp100: overall survival findings [abstract]. J Clin Oncol. 2018;36(15 Suppl):9521.
Sacco JJ, Carvajal R, Butler MO, et al. A phase (ph) II, multi-center study of the safety and efficacy of tebentafusp (tebe) (IMCgp100) in patients (pts) with metastatic uveal melanoma (mUM) [abstract no. 64MO]. Ann Oncol. 2020;31(Suppl 7):S1442–3.
Sacco J, Carvajal R, Butler M, et al. Updated survival of patients with previously treated metastatic uveal melanoma who received tebentafusp [abstract no. 538]. J Immunother Cancer. 2021;9(Suppl 2):A568.
Butler MO, Sato T, Carvajal RD, et al. Kinetics of radiographic response for tebentafusp (tebe) in previously treated metastatic uveal melanoma (mUM) patients (pts) achieving prolonged survival [abstract no. CT038]. Cancer Res. 2021;81(13 Suppl).
Hamid O, Hassel J, Shoushtari A, et al. Results from phase Ib study of tebentafusp (TEBE) in combination with durvalumab (DURVA) and/or tremelimumab (TREME) in metastatic cutaneous melanoma (MCM) [abstract no. 546]. J Immunother Cancer. 2021;9(Suppl 2):A576.
Middleton MR, McAlpine C, Woodcock VK, et al. Tebentafusp, A TCR/anti-CD3 bispecific fusion protein targeting gp100, potently activated antitumor immune responses in patients with metastatic melanoma. Clin Cancer Res. 2020;26(22):5869–78.
Salama AKS, Cheshuk V, Siveke J, et al. Characterization of cytokine release syndrome (CRS) following treatment with tebentafusp in previously untreated patients with metastatic uveal melanoma [abstract no. 1014P]. Ann Oncol. 2021;32(Suppl 5):S855.
Chmielowski B, Kapiteijn E, Ascierto PA, et al. Characterization of liver function tests following tebentafusp in phase III randomized trial comparing tebentafusp with investigator’s choice in first line metastatic uveal melanoma (mUM) [abstract no. 1018P]. Ann Oncol. 2021;32(Suppl 5):S856–7.
Carvajal RD, Sato T, Butler MO, et al. Characterization of cytokine release syndrome (CRS) following treatment with tebentafusp in patients (pts) with previously treated (2L+) metastatic uveal melanoma (mUM) [abstract]. J Clin Oncol. 2021;39(15 Suppl):9531.
Sato T, Carvajal RD, Sacco JJ, et al. Characterization of liver function tests (LFTs) following tebentafusp (tebe) in previously treated (2L+) metastatic uveal melanoma (mUM) patients (pts) [abstract]. J Clin Oncol. 2021;39(15 Suppl):e21513.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Sohita Dhillon is a contracted employee of Adis International Ltd/Springer Nature and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dhillon, S. Tebentafusp: First Approval. Drugs 82, 703–710 (2022). https://doi.org/10.1007/s40265-022-01704-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-022-01704-4