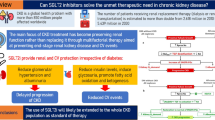
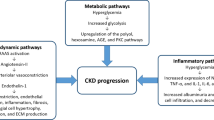
Abstract
Chronic kidney disease (CKD) is a serious, progressive condition associated with significant patient morbidity. Hypertension control and use of renin-angiotensin system blockers are the cornerstones of treatment for CKD. However, even with these treatment strategies, many individuals will progress towards kidney failure. Recently, sodium–glucose cotransporter 2 (SGLT2) inhibitor clinical trials with primary renal endpoints have firmly established SGLT2 inhibition, in addition to standard of care, as an effective strategy to slow down the progression of CKD and reduce some of its associated complications. The emergence of this new clinical evidence supports the use of SGLT2 inhibitors in the management of CKD in people with and without diabetes. As licensing and guidelines for SGLT2 inhibitors are updated, there is a need to adapt CKD treatment pathways and for this class of drugs to be included as part of standard care for CKD management. In this article, we have used consensus opinion alongside the available evidence to provide support for the healthcare community involved in CKD management, regarding the role of SGLT2 inhibitors in clinical practice. By highlighting appropriate prescribing and practical considerations, we aim to encourage greater and safe use of SGLT2 inhibitors for people with CKD, both with and without diabetes.

Similar content being viewed by others
References
Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR. Development and progression of nephropathy in type 2 diabetes: the United Kingdom prospective diabetes study (UKPDS 64). Kidney Int. 2003;63(1):225–32.
Chen Y, Lee K, Ni Z, He JC. Diabetic kidney disease: challenges, advances, and opportunities. Kidney Dis (Basel). 2020;6(4):215–25.
GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England). 2020;395(10225):709–33.
Segall L, Nistor I, Covic A. Heart failure in patients with chronic kidney disease: a systematic integrative review. Biomed Res Int. 2014;2014:937398.
Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143(11):1157–72.
Vanholder R, Massy Z, Argiles A, Spasovski G, Verbeke F, Lameire N. Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol Dial Transplant. 2005;20(6):1048–56.
Thompson S, James M, Wiebe N, Hemmelgarn B, Manns B, Klarenbach S, et al. Cause of death in patients with reduced kidney function. J Am Soc Nephrol. 2015;26(10):2504–11.
Disease K. Improving Global Outcomes (KDIGO) Diabetes Work Group KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020;98(4s):S1-s115.
National Institute for Health and Care Excellence. Clinical guideline [CG182]: Chronic kidney disease in adults: assessment and management 2014. Available from: https://www.nice.org.uk/guidance/cg182.
Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329(20):1456–62.
Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–60.
Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–9.
Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383(23):2219–29.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28.
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57.
Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–57.
Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383(15):1425–35.
Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–34.
Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Erondu N, Shaw W, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6(9):691–704.
Mosenzon O, Wiviott SD, Cahn A, Rozenberg A, Yanuv I, Goodrich EL, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7(8):606–17.
McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008.
Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–24.
Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–46.
U.S. Food and Drug Administration. FDA Approves Treatment for Chronic Kidney Disease2021. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-treatment-chronic-kidney-disease.
AstraZeneca UK Limited. Forxiga approved in the EU for the treatment of chronic kidney disease in patients with and without type-2 diabetes2021. Available from: https://www.astrazeneca.com/media-centre/press-releases/2021/forxiga-approved-in-the-eu-for-ckd.html.
AstraZeneca UK Limited. Forxiga approved in Japan for the treatment of chronic kidney disease in patients with and without type-2 diabetes2021. Available from: https://www.astrazeneca.com/media-centre/press-releases/2021/forxiga-approved-in-japan-for-ckd.html.
Škrtić M, Cherney DZ. Sodium-glucose cotransporter-2 inhibition and the potential for renal protection in diabetic nephropathy. Curr Opin Nephrol Hypertens. 2015;24(1):96–103.
Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet (London, England). 2020;396(10254):819–29.
Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2021;384(2):129–39.
Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–306.
Heerspink HJL, Oshima M, Zhang H, Li J, Agarwal R, Capuano G, et al. Canagliflozin reduces kidney-related adverse events in type 2 diabetes and ckd: findings from the randomized CREDENCE trial. Am J Kidney Dis. 2021;2:2.
Wheeler DC, Toto RD, Stefánsson BV, Jongs N, Chertow GM, Greene T, et al. A pre-specified analysis of the DAPA-CKD trial demonstrates the effects of dapagliflozin on major adverse kidney events in patients with IgA nephropathy. Kidney Int. 2021;100(1):215–24.
ClinicalTrials.gov. EMPA-KIDNEY (The Study of Heart and Kidney Protection With Empagliflozin) [Available from: https://www.clinicaltrials.gov/ct2/show/**NC**T***03594110.
Heerspink HJL, Karasik A, Thuresson M, Melzer-Cohen C, Chodick G, Khunti K, et al. Kidney outcomes associated with use of SGLT2 inhibitors in real-world clinical practice (CVD-REAL 3): a multinational observational cohort study. Lancet Diabetes Endocrinol. 2020;8(1):27–35.
Couser WG, Remuzzi G, Mendis S, Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011;80(12):1258–70.
Darlington O, Dickerson C, Evans M, McEwan P, Sörstadius E, Sugrue D, et al. Costs and healthcare resource use associated with risk of cardiovascular morbidity in patients with chronic kidney disease: evidence from a systematic literature review. Adv Ther. 2021;38(2):994–1010.
Wyld ML, Lee CM, Zhuo X, White S, Shaw JE, Morton RL, et al. Cost to government and society of chronic kidney disease stage 1–5: a national cohort study. Intern Med J. 2015;45(7):741–7.
Vupputuri S, Kimes TM, Calloway MO, Christian JB, Bruhn D, Martin AA, et al. The economic burden of progressive chronic kidney disease among patients with type 2 diabetes. J Diabetes Complications. 2014;28(1):10–6.
McQueen RB, Farahbakhshian S, Bell KF, Nair KV, Saseen JJ. Economic burden of comorbid chronic kidney disease and diabetes. J Med Econ. 2017;20(6):585–91.
Aggarwal HK, Jain D, Pawar S, Yadav RK. Health-related quality of life in different stages of chronic kidney disease. QJM. 2016;109(11):711–6.
Willis M, Nilsson A, Kellerborg K, Ball P, Roe R, Traina S, et al. Cost-effectiveness of canagliflozin added to standard of care for treating diabetic kidney disease (DKD) in patients with type 2 diabetes mellitus (T2DM) in England: estimates using the CREDEM-DKD model. Diabetes Ther. 2021;12(1):313–28.
McEwan P, Darlington O, Wheeler D, Heerspink H, Bergenheim K, Sanchez JG. POS-335: Cost-effectiveness of dapagliflozin as a treatment for chronic kidney disease: A health-economic analysis oF DAPA-CKD. Kidney Int Rep. 2021;6(4):S145–6.
McEwan P, Darlington O, Miller R, McMurray JJV, Wheeler DC, Heerspink HJL, et al. Cost-effectiveness of dapagliflozin in addition to current background therapy as a treatment for chronic kidney disease. . CJASN. 2021.
McEwan P, Darlington O, Boyce R, Heerspink HL, Wheeler DC, Garcia Sanchez JJ, editors. MO876: Estimating the budget impact of dapagliflozin for the treatment of chronic kidney disease from a UK payer perspective. ERA-EDTA; 2021; Virtual. .
Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C, et al. 2019 update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2020;63(2):221-8.
Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255–323.
EMC. SmPC: Forxiga 10 mg film-coated tablets2021. Available from: https://www.medicines.org.uk/emc/product/7607/smpc.
EMC. SmPC: Invokana 100 mg and 300 mg film-coated tables2020. Available from: https://www.medicines.org.uk/emc/product/8855/smpc.
Paul SK, Bhatt DL, Montvida O. The association of amputations and peripheral artery disease in patients with type 2 diabetes mellitus receiving sodium-glucose cotransporter type-2 inhibitors: real-world study. Eur Heart J. 2021;42(18):1728–38.
Mahling M, Schork A, Nadalin S, Fritsche A, Heyne N, Guthoff M. Sodium-glucose cotransporter 2 (SGLT2) inhibition in kidney transplant recipients with diabetes mellitus. Kidney Blood Press Res. 2019;44(5):984–92.
Schwaiger E, Burghart L, Signorini L, Ristl R, Kopecky C, Tura A, et al. Empagliflozin in posttransplantation diabetes mellitus: A prospective, interventional pilot study on glucose metabolism, fluid volume, and patient safety. Am J Transplant. 2019;19(3):907–19.
AlKindi F, Al-Omary HL, Hussain Q, Al Hakim M, Chaaban A, Boobes Y. Outcomes of SGLT2 inhibitors use in diabetic renal transplant patients. Transplant Proc. 2020;52(1):175–8.
Song CC, Brown A, Winstead R, Yakubu I, Demehin M, Kumar D, et al. Early initiation of sodium-glucose linked transporter inhibitors (SGLT-2i) and associated metabolic and electrolyte outcomes in diabetic kidney transplant recipients. Endocrinol Diabetes Metab. 2021;4(2):e00185.
Shah M, Virani Z, Rajput P, Shah B. Efficacy and safety of canagliflozin in kidney transplant patients. Indian J Nephrol. 2019;29(4):278–81.
Rajasekeran H, Kim SJ, Cardella CJ, Schiff J, Cattral M, Cherney DZI, et al. Use of Canagliflozin in kidney transplant recipients for the treatment of type 2 diabetes: a case series. Diabetes Care. 2017;40(7):e75–6.
Evans M, Hicks D, Patel D, Patel V, McEwan P, Dashora U. Optimising the benefits of SGLT2 inhibitors for type 1 diabetes. Diabetes Ther. 2020;11(1):37–52.
Ptaszynska A, Johnsson KM, Parikh SJ, de Bruin TW, Apanovitch AM, List JF. Safety profile of dapagliflozin for type 2 diabetes: pooled analysis of clinical studies for overall safety and rare events. Drug Saf. 2014;37(10):815–29.
Hirji I, Guo Z, Andersson SW, Hammar N, Gomez-Caminero A. Incidence of urinary tract infection among patients with type 2 diabetes in the UK General Practice Research Database (GPRD). J Diabetes Complications. 2012;26(6):513–6.
Wilke T, Boettger B, Berg B, Groth A, Mueller S, Botteman M, et al. Epidemiology of urinary tract infections in type 2 diabetes mellitus patients: an analysis based on a large sample of 456,586 German T2DM patients. J Diabetes Complications. 2015;29(8):1015–23.
Yavin Y, Mansfield TA, Ptaszynska A, Johnsson K, Parikh S, Johnsson E. Effect of the SGLT2 inhibitor dapagliflozin on potassium levels in patients with type 2 diabetes mellitus: a pooled analysis. Diabetes Ther. 2016;7(1):125–37.
Tang H, Zhang X, Zhang J, Li Y, Del Gobbo LC, Zhai S, et al. Elevated serum magnesium associated with SGLT2 inhibitor use in type 2 diabetes patients: a meta-analysis of randomised controlled trials. Diabetologia. 2016;59(12):2546–51.
Tsimihodimos V, Filippatos TD, Elisaf MS. Effects of sodium-glucose co-transporter 2 inhibitors on metabolism: unanswered questions and controversies. Expert Opin Drug Metab Toxicol. 2017;13(4):399–408.
Li X, Li T, Cheng Y, Lu Y, Xue M, Xu L, et al. Effects of SGLT2 inhibitors on fractures and bone mineral density in type 2 diabetes: an updated meta-analysis. Diabetes Metab Res Rev. 2019;35(7):e3170.
Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15(9):853–62.
Maruyama T, Takashima H, Oguma H, Nakamura Y, Ohno M, Utsunomiya K, et al. Canagliflozin improves erythropoiesis in diabetes patients with anemia of chronic kidney disease. Diabetes Technol Ther. 2019;21(12):713–20.
Oshima M, Neuen BL, Jardine MJ, Bakris G, Edwards R, Levin A, et al. Effects of canagliflozin on anaemia in patients with type 2 diabetes and chronic kidney disease: a post-hoc analysis from the CREDENCE trial. Lancet Diabetes Endocrinol. 2020;8(11):903–14.
Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2010;375(9733):2223–33.
Ferrannini E, Seman L, Seewaldt-Becker E, Hantel S, Pinnetti S, Woerle HJ. A Phase IIb, randomized, placebo-controlled study of the SGLT2 inhibitor empagliflozin in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15(8):721–8.
Wilding JP, Ferrannini E, Fonseca VA, Wilpshaar W, Dhanjal P, Houzer A. Efficacy and safety of ipragliflozin in patients with type 2 diabetes inadequately controlled on metformin: a dose-finding study. Diabetes Obes Metab. 2013;15(5):403–9.
Cefalu WT, Leiter LA, Yoon KH, Arias P, Niskanen L, Xie J, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet (London, England). 2013;382(9896):941–50.
Schork A, Saynisch J, Vosseler A, Jaghutriz BA, Heyne N, Peter A, et al. Effect of SGLT2 inhibitors on body composition, fluid status and renin-angiotensin-aldosterone system in type 2 diabetes: a prospective study using bioimpedance spectroscopy. Cardiovasc Diabetol. 2019;18(1):46.
Ohara K, Masuda T, Morinari M, Okada M, Miki A, Nakagawa S, et al. The extracellular volume status predicts body fluid response to SGLT2 inhibitor dapagliflozin in diabetic kidney disease. Diabetol Metab Syndr. 2020;12:37.
Masuda T, Watanabe Y, Fukuda K, Watanabe M, Onishi A, Ohara K, et al. Unmasking a sustained negative effect of SGLT2 inhibition on body fluid volume in the rat. Am J Physiol Renal Physiol. 2018;315(3):F653–64.
Dhatariya KK. Defining and characterising diabetic ketoacidosis in adults. Diabetes Res Clin Pract. 2019;155:107797.
Perlman A, Heyman SN, Matok I, Stokar J, Muszkat M, Szalat A. Acute renal failure with sodium-glucose-cotransporter-2 inhibitors: analysis of the FDA adverse event report system database. Nutr Metab Cardiovasc Dis. 2017;27(12):1108–13.
Oshima M, Jardine MJ, Agarwal R, Bakris G, Cannon CP, Charytan DM, et al. Insights from CREDENCE trial indicate an acute drop in estimated glomerular filtration rate during treatment with canagliflozin with implications for clinical practice. Kidney Int. 2021;99(4):999–1009.
Menne J, Dumann E, Haller H, Schmidt BMW. Acute kidney injury and adverse renal events in patients receiving SGLT2-inhibitors: A systematic review and meta-analysis. PLoS Med. 2019;16(12):e1002983.
Acknowledgements
We thank David Wheeler of the University College, London for reviewing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This manuscript was supported by a grant from AstraZeneca UK Ltd. in respect to medical writing and publication costs. AstraZeneca has not influenced the content of the publication, nor been involved in the design, collection, analysis, or reporting of any data presented. AstraZeneca UK Ltd. has reviewed this document for factual accuracy only.
Conflicts of interest
ME reports honoraria from AstraZeneca, Novo Nordisk, Takeda, and NAPP and research support from Novo Nordisk outside the submitted work. ARM is an employee of Health Economics and Outcomes Research Ltd., Cardiff, UK, who received fees from AstraZeneca in relation to this study. MBW reports investigator-led research grants from Sanofi, Eli Lilly, and AstraZeneca and personal fees from AstraZeneca, Boehringer Ingelheim, and MSD outside the submitted work. WH reports grants and personal fees from AstraZeneca, grants and personal fees from Boehringer Ingelheim, grants and personal fees from NAPP, and from MSD, outside the submitted work. SCB reports personal fees and other from Abbott, personal fees and other from AstraZeneca, personal fees and other from Boehringer Ingelheim, personal fees and other from Eli Lilly, personal fees and other from Merck Sharp & Dohme, personal fees and other from Novo Nordisk, personal fees and other from Sanofi-Aventis, other from Cardiff University, other from Doctors.net, other from Elsevier, other from OnMedica, other from OmniaMed, other from Medscape, other from All-Wales Medicines Strategy Group, other from National Institute for Health and Care Excellence (NICE) UK, and other from Glycosmedia, outside the submitted work. PAK reports personal fees for lecturing from Astra Zeneca, Boehringer Ingelheim, NAPP, MundiPharma, and Novo Nordisk outside the submitted work. SD has received honorarium from AstraZeneca, Boehringer Ingelheim, Lilly, Novo Nordisk, Takeda, MSD, NAPP, Bayer, and Roche for attending and participating in educational events and advisory boards, outside the submitted work. UD reports personal fees from AstraZeneca, Napp, Sanofi, BI, Lilly, and Novo Nordisk, outside the submitted work. ZY reports personal fees from AstraZeneca, personal fees from Lilly, personal fees from Boehringer Ingelheim, and personal fees from Novartis outside the submitted work. DCP reports personal fees from AstraZeneca, personal fees from Boehringer Ingelheim, personal fees from Eli Lilly, non-financial support from Napp, personal fees from Novo Nordisk, personal fees from MSD, and personal fees and non-financial support from Sanofi outside the submitted work. In addition, DCP is an executive committee member of the Association of British Clinical Diabetologists and member of the CaReMe UK group. WDS holds research grants from Bayer, Novo Nordisk, and Novartis and has received speaker honoraria from AstraZeneca, Bayer, Bristol-Myers Squibb, Merck, Napp, Novartis, Novo Nordisk, and Takeda. WDS is supported by the NIHR Exeter Clinical Research Facility and the NIHR Collaboration for Leadership in Applied Health Research and Care (CLAHRC) for the South West Peninsula.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
Author contribution
All authors have been involved in the design of this review. Marc Evans and Angharad R Morgan produced the primary manuscript. All authors have contributed to the drafting and revision of the manuscript. All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Rights and permissions
About this article
Cite this article
Evans, M., Morgan, A.R., Whyte, M.B. et al. New Therapeutic Horizons in Chronic Kidney Disease: The Role of SGLT2 Inhibitors in Clinical Practice. Drugs 82, 97–108 (2022). https://doi.org/10.1007/s40265-021-01655-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-021-01655-2