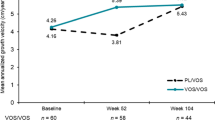
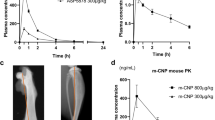
Abstract
Vosoritide (VOXZOGO®) is a modified recombinant human C-type natriuretic peptide (CNP) analogue, being developed by BioMarin Pharmaceutical for the treatment of achondroplasia. Achondroplasia is caused by a gain-of-function mutation in the fibroblast growth factor receptor 3 gene (FGFR3), which is a negative regulator of bone growth. Vosoritide acts to restore chondrogenesis through its binding to natriuretic peptide receptor B (NPR-B), resulting in the inhibition of downstream signalling pathways of the overactive FGFR3 gene. Vosoritide was approved in August 2021 in the EU for the treatment of achondroplasia in patients aged ≥ 2 years whose epiphyses are not closed; the diagnosis of achondroplasia should be confirmed by appropriate genetic testing. The drug is also under regulatory review in the USA for the treatment of achondroplasia and clinical development is underway in several countries. This article summarizes the milestones in the development of vosoritide leading to this first approval for achondroplasia in patients aged ≥ 2 years whose epiphyses are not closed.
Similar content being viewed by others
References
Savarirayan R, Irving M, Bacino CA, et al. C-Type natriuretic peptide analogue therapy in children with achondroplasia. N Engl J Med. 2019;381(1):25–35.
Semler O, Rehberg M, Mehdiani N, et al. Current and emerging therapeutic options for the management of rare skeletal diseases. Paediatr Drugs. 2019;21(2):95–106.
Wrobel W, Pach E, Ben-Skowronek I. Advantages and disadvantages of different treatment methods in achondroplasia: a review. Int J Mol Sci. 2021;22(11):5573. https://doi.org/10.3390/ijms22115573.
Wendt DJ, Dvorak-Ewell M, Bullens S, et al. Neutral endopeptidase-resistant C-type natriuretic peptide variant represents a new therapeutic approach for treatment of fibroblast growth factor receptor 3-related dwarfism. J Pharmacol Exp Ther. 2015;353(1):132–49.
Chan ML, Qi Y, Larimore K, et al. Pharmacokinetics and exposure-response of vosoritide in children with achondroplasia. Clin Pharmacokinet. 2021. https://doi.org/10.1007/s40262-021-01059-1.
BioMarin International Limited. Voxzogo: EU summary of product characteristics. 2021. https://ec.europa.eu/health/documents/community-register/2021/20210826152503/anx_152503_en.pdf. Accessed 20 Sep 2021.
BioMarin Pharmaceutical. European Commission approves BioMarin's VOXZOGO® (vosoritide) for the treatment of children with achondroplasia from age 2 until growth plates close [media release]. 27 Aug 2021. https://investors.biomarin.com/.
BioMarin Pharmaceutical. BioMarin submits new drug application to U.S. Food and Drug Administration for vosoritide to treat children with achondroplasia [media release]. 21 Aug 2020. http://www.biomarin.com.
Chugai P. Chugai and BioMarin enter exclusive sublicense agreement on the patent of C- type natriuretic peptide intended for treatment of achondroplasia [media release]. 3 Mar 2016. http://www.chugai-pharm.co.jp.
Lorget F, Kaci N, Peng J, et al. Evaluation of the therapeutic potential of a CNP analog in a Fgfr3 mouse model recapitulating achondroplasia. Am J Hum Genet. 2012;91(6):1108–14.
Savarirayan R, Tofts L, Irving M, et al. Once-daily, subcutaneous vosoritide therapy in children with achondroplasia: a randomised, double-blind, phase 3, placebo-controlled, multicentre trial. Lancet. 2020;396(10252):684–92.
Hoover-Fong J, Irving M, Bacino C, et al. Vosoritide for children with achondroplasia: a 60-month update from an ongoing phase 2 clinical trial. Mol Genet Metab. 2021;132(Suppl 1):S101.
Savarirayan R, Tofts L, Irving M et al. Safe and persistent growth-promoting effects of vosoritide in children with achondroplasia: 2-year results from an open-label, phase 3 extension study. Genet Med. 2021. https://doi.org/10.1038/s41436-021-01287-7
Savarirayan R, Irving M, Maixner W, et al. Rationale, design, and methods of a randomized, controlled, open-label clinical trial with open-label extension to investigate the safety of vosoritide in infants, and young children with achondroplasia at risk of requiring cervicomedullary decompression surgery. Sci Prog. 2021;104(1):368504211003782.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Sean Duggan is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Duggan, S. Vosoritide: First Approval. Drugs 81, 2057–2062 (2021). https://doi.org/10.1007/s40265-021-01623-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-021-01623-w