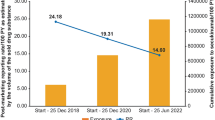
Abstract
Hetrombopag (Hengqu®), an oral nonpeptide thrombopoietin receptor agonist, is being developed by Jiangsu Hengrui Pharmaceutical for the treatment of thrombocytopenia and aplastic anaemia. On 16 June 2021, hetrombopag received its first approval in China as a second-line treatment for primary immune thrombocytopenia (ITP) and severe aplastic anaemia (SAA) in adults. The drug is also undergoing phase III development in China for the treatment of chemotherapy-induced thrombocytopenia. This article summarizes the milestones in the development of hetrombopag leading to this first approval for ITP and SAA.
Similar content being viewed by others
References
Audia S, Mahevas M, Samson M, et al. Pathogenesis of immune thrombocytopenia. Autoimmun Rev. 2017;16(6):620–32.
Mei H, Liu X, Li Y, et al. A multicenter, randomized phase III trial of hetrombopag: a novel thrombopoietin receptor agonist for the treatment of immune thrombocytopenia. J Hematol Oncol. 2021;14(1):37.
Killick SB, Bown N, Cavenagh J, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172(2):187–207.
Xie C, Zhao H, Bao X, et al. Pharmacological characterization of hetrombopag, a novel orally active human thrombopoietin receptor agonist. J Cell Mol Med. 2018;22(11):5367–77.
Hengrui JS. Hetrombopag olamine tablets: Chinese prescribing information. 2021
National Medical Products Administration. 2021. https://www.nmpa.gov.cn/zwfw/sdxx/sdxxyp/yppjfb/20210621084907105.html. Accessed 19 Jul 2021
Zheng L, Liang MZ, Zeng XL, et al. Safety, pharmacokinetics and pharmacodynamics of hetrombopag olamine, a novel TPO-R agonist, in healthy individuals. Basic Clin Pharmacol Toxicol. 2017;121(5):414–22.
Wang Z, Chen X, Li A, et al. Effect of food on the pharmacokinetic and pharmacodynamic profiles of hetrombopag in healthy volunteers. Clin Ther. 2020;42(12):2280–8.
Yang G, Huang R, Yang S, et al. Effect of postdose fasting duration on hetrombopag olamine pharmacokinetics and pharmacodynamics in healthy volunteers. Br J Clin Pharmacol. 2020;86(8):1528–36.
Wang Z, Chen L, Zhang F, et al. First-in-patient study of hetrombopag in patients with chronic idiopathic thrombocytopenic purpura. J Thromb Haemost. 2020;18(11):3053–60.
Zhang F, Peng G, He G, et al. A multicenter, open-label, single-arm, phase 2 study to evaluate the efficacy and safety of hetrombopag in patients with severe aplastic anemia (SAA) [abstract no. 2751]. Blood. 2020;136(Suppl. 1):3–4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Yahiya Y. Syed is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Syed, Y.Y. Hetrombopag: First Approval. Drugs 81, 1581–1585 (2021). https://doi.org/10.1007/s40265-021-01575-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-021-01575-1