Abstract
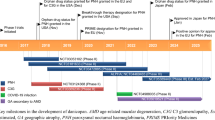
Pegcetacoplan (Empaveli™) is a PEGylated pentadecapeptide developed by Apellis Pharmaceuticals for the treatment of complement-mediated diseases. It binds to complement component 3 (C3) and its activation fragment C3b, controlling the cleavage of C3 and the generation of the downstream effectors of complement activation and thus both C3b-mediated extravascular haemolysis and terminal complement-mediated intravascular haemolysis. Pegcetacoplan is the first C3-targeted paroxysmal nocturnal haemoglobinuria (PNH) therapy to be approved (in May 2021) in the USA, where it is indicated for the treatment of adults with PNH, including those switching from C5 inhibitor therapy with eculizumab and ravulizumab. A regulatory assessment of pegcetacoplan for the treatment of PNH is currently underway in the EU and Australia. Pegcetacoplan is also being investigated as a therapeutic option in other complement-mediated diseases, including age-related macular degeneration, C3 glomerulopathy and autoimmune haemolytic anaemia. The recommended dosage regimen of pegcetacoplan is 1080 mg twice weekly, administered as a subcutaneous infusion via an infusion pump with a ≥ 20 mL reservoir. This article summarizes the milestones in the development of pegcetacoplan leading to this first approval for the treatment of adults with PNH.
Similar content being viewed by others
References
US Library of Medicine. Genetics home reference: paroxysmal nocturnal hemoglobinuria. 2021. https://medlineplus.gov/genetics/condition/paroxysmal-nocturnal-hemoglobinuria/. Accessed 3 June 2021.
Hill A, DeZern AE, Kinoshita T, et al. Paroxysmal nocturnal haemoglobinuria. Nat Rev Dis Prim. 2017;3:17028.
Inoue N, Izui-Sarumaru T, Murakami Y, et al. Molecular basis of clonal expansion of hematopoiesis in 2 patients with paroxysmal nocturnal hemoglobinuria (PNH). Blood. 2006;108(13):4232–6.
Risitano AM, Marotta S, Ricci P, et al. Anti-complement treatment for paroxysmal nocturnal hemoglobinuria: time for proximal complement inhibition? A position paper from the SAAWP of the EBMT. Front Immunol. 2019;10:1157.
Apellis Pharmaceuticals. EMPAVELI™ (pegcetacoplan) injection, for subcutaneous use: US prescribing information. 2021. https://pi.apellis.com/files/PI_Empaveli.pdf. Accessed 17 May 2021.
Apellis Pharmaceuticals. Apellis announces U.S. Food and Drug Administration (FDA) approval of EMPAVELI™ (pegcetacoplan) for adults with paroxysmal nocturnal hemoglobinuria (PNH) [media release]. 14 May 2021. http://www.apellis.com/.
US Food & Drug Administration. FDA approves new treatment for adults with serious rare blood disease [media release]. 14 May 2021. https://www.fda.gov/
Apellis Pharmaceuticals. Apellis Pharmaceuticals reports third quarter 2020 financial results [media release]. 2 Nov 2020. http://www.apellis.com.
Apellis Pharmaceuticals. Apellis Pharmaceuticals' APL-2 receives orphan drug designation from the FDA for the treatment of C3 glomerulopathy [media release]. 20 Dec 2018. http://www.apellis.com.
European Medicines Agency. Public summary of opinion on orphan designation. 2019. https://www.ema.europa.eu/. Accessed 4 Jun 2021
Apellis Pharmaceuticals. Apellis’ APL-2 receives orphan drug designation from the FDA for the treatment of autoimmune hemolytic anemia [media release]. 5 Feb 2019. http://www.apellis.com
Apellis Pharmaceuticals. Securities and Exchange Commission Form 10-K. 2021. https://investors.apellis.com/static-files/9ff4931a-086c-46d0-b296-ac3ba9f93276. Accessed 9 Jun 2021.
Apellis Pharmaceuticals. Apellis Pharmaceuticals announces collaboration with SFJ Pharmaceuticals(Rm) for APL-2 in hematologic indications [media release]. 28 Feb 2019. http://www.apellis.com.
Apellis Pharmaceuticals, Sobi. Apellis and Sobi enter collaboration for global co-development and ex-US commercialization of systemic pegcetacoplan in rare diseases with urgent need for new treatments [media release]. 27 Oct 2020. http://www.apellis.com.
Simon-Tillaux N, Chauvet S, El Mehdi D, et al. APL-2 prevents both C3 and C5 convertase formation and activity: a potential therapeutic for renal diseases [abstract no. SA-PO 609]. J Am Soc Nephrol. 2019;30:918.
Hillmen P, Szer J, Weitz I, et al. Pegcetacoplan versus eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2021;384(11):1028–37.
Swedish Orphan Biovitrum. Sobi and Apellis report positive top-line results at 48 weeks from the phase 3 PEGASUS study of pegcetacoplan in PNH [media release]. 10 Dec 2020. http://www.sobi.com.
Apellis Pharmaceuticals, Sobi. Apellis and Sobi report positive top-line results from the phase 3 PRINCE study of EMPAVELI™ (pegcetacoplan) in treatment-naïve patients with PNH [media release]. 25 May 2021. http://www.apellis.com/.
Liao DS, Grossi FV, El Mehdi D, et al. Complement C3 inhibitor pegcetacoplan for geographic atrophy secondary to age-related macular degeneration: a randomized phase 2 trial. Ophthalmology. 2020;127(2):186–95.
Dixon BP, Greenbaum LA, Huang L, et al. C3 inhibition with pegcetacoplan targets the underlying disease process of C3 glomerulopathy (C3G) and improves proteinuria [abstract no. PO1852 plus poster]. J Am Soc Nephrol. 2020;31:577.
Apellis Pharmaceuticals. Apellis to present positive results from the phase 2 DISCOVERY study of pegcetacoplan in C3 glomerulopathy (C3G) at ASN Kidney Week [media release]. 9 Oct 2020. http://www.apellis.com.
Gertz M, Roman E, Fattizzo B, et al. Inhibition of C3 with APL-2 controls haemolysis and increases haemoglobin levels in subjects with autoimmune haemolytic anaemia (AIHA) [abstract no. BSH19-OR-024]. Br J Haematol. 2019;185(Suppl 1):24.
Apellis Pharmaceuticals. Apellis Pharmaceuticals presents data from ongoing APL-2 phase 2 study in patients with cold agglutinin disease and warm antibody autoimmune hemolytic anemia at 24th European Hematology Association (EHA) Congress [media release]. 15 Jun 2019. http://www.apellis.com.
Wykoff CC, Rosenfeld PJ, Waheed NK, et al. Characterizing new-onset exudation in the randomized phase 2 FILLY trial of complement inhibitor pegcetacoplan for geographic atrophy. Ophthalmology. 2021. https://doi.org/10.1016/j.ophtha.2021.02.025
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Sheridan Hoy is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hoy, S.M. Pegcetacoplan: First Approval. Drugs 81, 1423–1430 (2021). https://doi.org/10.1007/s40265-021-01560-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-021-01560-8