Abstract
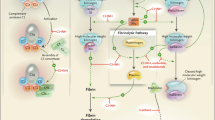
Berotralstat (ORLADEYO™) is an orally administered kallikrein inhibitor, which has been developed by BioCryst Pharmaceuticals for hereditary angioedema (HAE). The inhibition of kallikrein by berotralstat decreases the production of bradykinin, which prevents the localised tissue oedema that occurs during attacks of HAE. Berotralstat has been approved in the USA, and subsequently in Japan, for prophylaxis to prevent attacks of HAE in adults and paediatric patients aged 12 years or older. This article summarises the milestones in the development of berotralstat leading to this first approval for prophylaxis to prevent attacks of HAE.
Similar content being viewed by others
References
Levi M, Cohn DM, Zeerleder S. Hereditary angioedema: linking complement regulation to the coagulation system. Res Pract Thromb Haemost. 2019;3(1):38–43.
BioCryst Pharmaceuticals. BioCryst announces FDA approval of ORLADEYO™ (berotralstat), first oral, once-daily therapy to prevent attacks in hereditary angioedema patients [media release]. 3 Dec 2020. https://ir.biocryst.com/.
BioCryst. Berotralstat (ORLADEYO™): US prescribing information. 2020. https://orladeyo.com/. Accessed 21 Jan 2020.
BioCryst Pharmaceuticals. BioCryst announces approval of ORLADEYO™ (berotralstat) in Japan for the prophylactic treatment of hereditary angioedema [media release]. 22 Jan 2020. https://ir.biocryst.com/.
Torii Pharmaceutical. Berotralstat (ORLADEYO™): Japanese prescribing information [Japanese]. 2021. https://www.pmda.go.jp. Accessed 2 Feb 2021.
Longhurst HJ, Stobiecki M, Zanichelli A, et al. Final results from the ZENITH-1 study: oral administration of plasma kallikrein inhibitor BCX7353 for the treatment of attacks in patients with hereditary angioedema [abstract no. OA0015]. Allergy. 2019;74(Suppl 106):10-11.
BioCryst Pharmaceuticals. BioCryst announces partnership with Torii Pharmaceutical to commercialize BCX7353 in Japan for the prevention of HAE attacks [media release]. 5 Nov 2019. https://ir.biocryst.com/.
Chen X, Kotian P, Wilson R, et al. Preclinical characterization of BCX7353, an oral plasma kallikrein inhibitor, for the treatment of hereditary angioedema (HAE) [abstract no. 722]. J Allergy Clin Immunol. 2017;139(Suppl 2):AB230.
Zuraw B, Lumry WR, Johnston DT, et al. Oral once-daily berotralstat for the prevention of hereditary angioedema attacks: a randomized, double-blind, placebo-controlled phase 3 trial. J Allergy Clin Immunol. 2020. https://doi.org/10.1016/j.jaci.2020.10.015.
Wedner H, Zuraw B, Anderson J, et al. Berotralstat reduces attacks in patients with hereditary angioedema (HAE): APeX-2 trial 48 week results [abstract no. D102]. Ann Allergy Asthma Immunol. 2020;125(Suppl 5):S14.
Ohsawa I, Honda D, Suzuki Y, et al. Oral berotralstat for the prophylaxis of hereditary angioedema attacks in patients in Japan: a phase 3 randomized trial. Allergy. 2020. https://doi.org/10.1111/all.14670.
Reshef A, Maurer M, Kiani S, et al. Long-term effectiveness of berotralstat (BCX7353) for the prophylaxis of hereditary angioedema (HAE) attacks: results from the APeX-S study [abstract no. 1406]. Allergy. 2020;75(Suppl 109):91–2.
Aygoren-Pursun E, Bygum A, Grivcheva-Panovska V, et al. Oral plasma kallikrein inhibitor for prophylaxis in hereditary angioedema. N Engl J Med. 2018;379(4):352–62.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. A. Lee is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Lee, A. Berotralstat: First Approval. Drugs 81, 405–409 (2021). https://doi.org/10.1007/s40265-021-01475-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-021-01475-4