Abstract
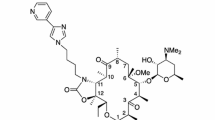
Lefamulin is a novel oral and intravenous (IV) pleuromutilin developed as a twice-daily treatment for community-acquired bacterial pneumonia (CABP). It is a semi-synthetic pleuromutilin with a chemical structure that contains a tricyclic core of five-, six-, and eight-membered rings and a 2-(4-amino-2-hydroxycyclohexyl)sulfanylacetate side chain extending from C14 of the tricyclic core. Lefamulin inhibits bacterial protein synthesis by binding to the 50S bacterial ribosomal subunit in the peptidyl transferase center (PTC). The pleuromutilin tricyclic core binds to a pocket close to the A site, while the C14 side chain extends to the P site causing a tightening of the rotational movement in the binding pocket referred to as an induced-fit mechanism. Lefamulin displays broad-spectrum antibacterial activity against Gram-positive and Gram-negative aerobic and anaerobic bacteria as well as against atypical bacteria that commonly cause CABP. Pleuromutilin antibiotics exhibit low rates of resistance development and lack cross-resistance to other antimicrobial classes due to their unique mechanism of action. However, pleuromutilin activity is affected by mutations in 23S rRNA, 50S ribosomal subunit proteins rplC and rplD, ATP-binding cassette (ABC)-F transporter proteins such as vga(A), and the methyltransferase cfr. The pharmacokinetic properties of lefamulin include: volume of distribution (Vd) ranging from 82.9 to 202.8 L, total clearance (CLT) of 19.5 to 21.4 L/h, and terminal elimination half-life (t1/2) of 6.9–13.2 h; protein binding of lefamulin is high and non-linear. The oral bioavailability of lefamulin has been estimated as 24% in fasted subjects and 19% in fed subjects. A single oral dose of lefamulin 600 mg administered in fasted patients achieved a maximum plasma concentration (Cmax) of 1.2–1.5 mg/L with a time of maximum concentration (Tmax) ranging from 0.8 to 1.8 h, and an area under the plasma concentration-time curve from 0 to infinity (AUC0−∞) of 8.5–8.8 mg h/L. The pharmacodynamic parameter predictive of lefamulin efficacy is the free plasma area under the concentration-time curve divided by the minimum inhibitory concentration (fAUC24h/MIC). Lefamulin efficacy has been demonstrated using various animal models including neutropenic murine thigh infection, pneumonia, lung infection, and bacteremia. Lefamulin clinical safety and efficacy was investigated through a Phase II clinical trial of acute bacterial skin and skin structure infection (ABSSSI), as well as two Phase III clinical trials of CABP. The Phase III trials, LEAP 1 and LEAP 2 established non-inferiority of lefamulin to moxifloxacin in both oral and IV formulations in the treatment of CABP. The United States Food and Drug Administration (FDA), European Medicines Agency (EMA), and Health Canada have each approved lefamulin for the treatment of CABP. A Phase II clinical trial has been completed for the treatment of ABSSSI, while the pediatric program is in Phase I. The most common adverse effects of lefamulin include mild-to-moderate gastrointestinal-related events such as nausea and diarrhea. Lefamulin represents a safe and effective option for treating CABP in cases of antimicrobial resistance to first-line therapies, clinical failure, or intolerance/adverse effects to currently used agents. Clinical experience and ongoing clinical investigation will allow clinicians and antimicrobial stewardship programs to optimally use lefamulin in the treatment of CABP.



Similar content being viewed by others
References
Dillon C, Guarascio AJ, Covvey JR. Lefamulin: a promising new pleuromutilin antibiotic in the pipeline. Expert Rev Anti Infect Ther. 2019;17(1):5–15.
Lee YR, Jacobs KL. Leave it to lefamulin: a pleuromutilin treatment option in community-acquired bacterial pneumonia. Drugs. 2019;79(17):1867–76.
Medical Association A. Lefamulin (Xenleta) for community-acquired bacterial pneumonia. Med Lett Drugs Ther. 2019;61(1581):145–8.
Paukner S, Riedl R. Pleuromutilins: potent drugs for resistant bugs-mode of action and resistance. Cold Spring Harb Perspect Med. 2017;7(1):1–15.
Novak R. Are pleuromutilin antibiotics finally fit for human use? Ann N Y Acad Sci. 2011;1241:71–81.
Falcó V, Burgos J, Almirante B. An overview of lefamulin for the treatment of community acquired bacterial pneumonia. Expert Opin Pharmacother. 2020;21(6):629–36.
Nabriva Therapeutics Announces European Medicines Agency (EMA). Validation of marketing authorization application for Lefamulin Nasdaq:NBRV. https://www.globenewswire.com/news-release/2019/06/24/1873292/0/en/Nabriva-Therapeutics-Announces-European-Medicines-Agency-EMA-Validation-of-Marketing-Authorization-Application-for-Lefamulin.html. Accessed 25 Apr 2020
Veve MP, Wagner JL. Lefamulin: review of a promising novel pleuromutilin antibiotic. Pharmacotherapy. 2018;38(9):935–46.
Novel Antibiotics | Lefamulin | Nabriva.com. https://www.nabriva.com/pipeline-research. Accessed 26 Apr 2020
Yi Y, Fu Y, Dong P, Qin W, Liu Y, Liang J, et al. Synthesis and biological activity evaluation of novel heterocyclic pleuromutilin derivatives. Molecules. 2017;22:996.
Eyal Z, Matzov D, Krupkin M, Wekselman I, Paukner S, Zimmerman E, et al. Structural insights into species-specific features of the ribosome from the pathogen staphylococcus aureus. Proc Natl Acad Sci USaA. 2015;112:E5805–14.
Li Y-G, Wang J-X, Zhang G-N, Zhu M, You X-F, Wang Y-C, et al. Design, synthesis, and biological activity evaluation of a series of pleuromutilin derivatives with novel C14 side chains. Bioorg Med Chem Lett. 2020;112:5905–14.
Susanne P, Astrid G, Rosemarie R. Lefamulin selectively inhibits bacterial protein synthesis [abstract no EP-0405 plus poster]. In: European congress of clinical microbiology and infectious diseases. 22–25 April 2017; Vienna.
Wang X, Ling Y, Wang H, Yu J, Tang J, Zheng H, et al. Novel pleuromutilin derivatives as antibacterial agents: synthesis, biological evaluation and molecular docking studies. Bioorg Med Chem Lett. 2012;22:6166–72.
Eyal Z, Matzov D, Krupkin M, Paukner S, Riedl R, Rozenberg H, et al. A novel pleuromutilin antibacterial compound, its binding mode and selectivity mechanism. Sci Rep. 2016;6:39004.
Mechanism of Injury [Internet]. US Nat Lib Med. 2019. https://www.accessdata.fda.gov/drugsatfda_docs/nda/. Accessed 28 May 2020.
Mendes RE, Paukner S, Doyle TB, Gelone SP, Flamm RK, Sader HS. Low prevalence of Gram-positive isolates showing elevated lefamulin MIC results during the sentry surveillance program for 2015–2016 and characterization of resistance mechanisms. Antimicrob Agents Chemother. 2019;63(4):e02158-e2218.
Perry W, Golan Y. Therapeutic potential of lefamulin in the treatment of community-acquired pneumonia. Future Microbiol. 2019;14:927–39.
Mendes RE, Paukner S, Doyle TB, Flamm RK, Sader HS. Low prevalence of gram-positive isolates showing elevated lefamulin MIC results during the SENTRY surveillance program for 2015–2016 and characterization of resistance mechanisms. Antimicrob Agents Chemother. 2019;63(4):1–9.
Putnam SD, Biedenbach DJ, Sader HS, Ivezic-Schoenfeld Z, Paukner S, Novak R, et al. Antimicrobial activity of the investigational pleuromutilin compound BC-3781 against Gram-positive organisms commonly associated with cutaneous infections [abstract no P910 plus poster]. In: European congress of clinical microbiology and infectious diseases. ECCMID 2010; Vienna.
Paukner S, Kollmann H, Thirring K, Heilmayer W, Ivezic-Schoenfeld Z. Antibacterial in vitro activity of novel extended spectrum pleuromutilins against Gram-positive and-negative bacterial pathogens [abstract no P1678 plus poster]. In: European congress of clinical microbiology and infectious diseases. 10–13 May 2014; Barcelona.
Paukner S, Wicha WW, Thirring K, Kollmann H, Ivezic-Schoenfeld Z. In vitro and in vivo efficacy of novel extended spectrum pleuromutilins against S. aureus and S. pneumoniae [abstract no P0247 plus poster]. In: European congress of clinical microbiology and infectious diseases. 25–28 April 2015; Denmark.
Paukner S, Sader HS, Streit J, Flamm RK, Gelone SP. In vitro activity of lefamulin against bacterial pathogens commonly causing community-acquired bacterial pneumonia (cap): 2015 SENTRY data from Europe [abstract no P1331 plus poster]. In: European congress of clinical microbiology and infectious diseases. 22–25 April 2017; Vienna.
Paukner S, Sader HS, Flamm R, Duncan L, Gelone SP. In vitro activity of lefamulin against S. aureus from hospital-acquired pneumonia (HAP) and community-acquired pneumonia (CAP) patients in Europe [abstract no P1332 plus poster]. In: European congress of clinical microbiology and infectious diseases. 22–25 April 2017; Vienna.
Paukner S, Flamm R, Streit J, Gelone SP, Sader HS. In vitro activity of lefamulin against S. aureus collected from hospitalized patients with bacterial pneumonia (cap) in Europe [abstract no P1333 plus poster]. In: European congress of clinical microbiology and infectious diseases. 22–25 April 2017; Vienna.
Paukner S, Sader HS, Streit JM, Flamm RK, Gelone SP. In vitro activity of lefamulin against bacterial pathogens commonly causing pneumonia collected from patients in Asia (2015) [abstract no FRIDAY 345 plus poster]. In: American society for microbiology MICROBE. 1–5 June 2017; New Orleans.
Paukner S, Flamm RK, Ryan Arends SJ, Gelone SP, Sader HS. In vitro activity of lefamulin against a colletion of respiratory pathogens from pediatric patients in US (SENTRY surveillance 2015) [abstract no FRIDAY 24 plus poster]. In: American society for microbiology MICROBE. 1–5 June 2017; New Orleans.
Paukner S, Sader HS, Streit JM, Flamm RK, Gelone SP. In vitro activity of lefamulin against bacterial pathogens collected from patients with community-acquired bacterial pneumonia (CABP) SENTRY 2015 US data [abstract no FRIDAY 85 plus poster]. In: American society for microbiology MICROBE. 1–5 June 2017; New Orleans.
Paukner S, Flamm R, Duncan L, Gelone SP, Sader HS. In vitro activity of lefamulin against global collection of respiratory pathogens from paediatric patients from the 2015 SENTRY program [abstract no P1334 plus poster]. In: European congress of clinical microbiology and infectious diseases. 22–25 April 2017; Vienna.
Paukner S, Flamm RK, Ryan Arends SJ, Gelone SP, Sader HS. In vitro antibacterial activity of lefamulin against S. aureus collected from hospitalized patients with bacterial pneumonia in US [abstract no FRIDAY 344 plus poster]. In: American society for microbiology MICROBE. 1–5 June 2017; New Orleans.
Paukner S, Gelone SP, Ryan Arends SJ, Flamm RK, Sader HS. Antibacterial activity of lefamulin against pathogens most commonly causing community-acquired bacterial pneumonia: SENTRY antimicrobial surveillance program (2015–2016). Antimicrob Agents Chemother. 2019;63(4):e02161-e2218.
Sader HS, Biedenbach DJ, Paukner S, Ivezic-Schoenfeld Z, Jones RN. Antimicrobial activity of the investigational pleuromutilin compound BC-3781 tested against Gram-positive organisms commonly associated with acute bacterial skin and skin structure infections. Antimicrob Agents Chemother. 2012;56(3):1619–23.
Mendes RE, Farrell DJ, Flamm RK, Talbot GH, Ivezic-Schoenfeld Z, Paukner S, et al. In vitro activity of lefamulin tested against Streptococcus pneumoniae with defined serotypes, including multidrug-resistant isolates causing lower respiratory tract infections in the United States. Antimicrob Agents Chemother. 2016;60(7):4407–11.
Paukner S, Gelone SP, Arends SJR, Sader HS. Lefamulin activity against bacterial pathogens commonly associated with acute bacterial skin and skin structure infections (ABSSSI) collected in the 2017 global SENTRY antimicrobial surveillance program [abstract no Saturday AAR-785 plus poster]. In: American society for microbiology MICROBE. 20–24 June 2019; San Francisco.
Alexander E, Goldberg L, Das AF, Sandrock C, Paukner S, Schranz J, et al. Efficacy of lefamulin (lef) versus moxifloxacin (mox) by pathogen in adults with community-acquired bacterial pneumonia (CABP): pooled results from the LEAP 1 and LEAP 2 Phase 3 clinical trials [abstract no Sunday CIV-177 plus poster]. In: American society for microbiology MICROBE. 20–24 June 2019; San Francisco.
Paukner S, Streit JM, Flamm RK, Gelone SP, Sader HS. In vitro activity of lefamulin against bacterial pathogens commonly causing acute bacterial skin and skin structure infections (ABSSSI) and bloodstream infections (BSI): Global SENTRY surveillance 2016 [abstract no Saturday 643 plus poster]. In: American society for microbiology MICROBE. 7–11 June 2018; Atlanta.
Paukner S, Streit JM, Flamm RK, Gelone SP, Sader HS. In vitro activity of lefamulin against bacterial pathogens commonly causing community-acquired respiratory tract infections (CARTI) global SENTRY surveillance 2016 [abstract no Saturday 644 plus poster]. In: American society for microbiology MICROBE. 7–11 June 2018; Atlanta.
Zhanel GG, Lam A, Schweizer F, Thomson K, Walkty A, Rubinstein E, et al. Ceftobiprole a review of a broad-spectrum and anti-MRSA cephalosporin. Am J Clin Dermatol. 2008;9(4):245–54.
Paukner S, Sader HS, Mendes RE, Flamm RK, Gelone SP. In vitro activity of lefamulin against bacterial pathogens collected from patients with community- or hospital-acquired respiratory tract infections: 2016 SENTRY data from Europe [abstract no P0619 plus poster]. In: European congress of clinical microbiology and infectious diseases. 21–24 April 2018; Madrid.
Paukner S, Flamm RK, Mendes RE, Gelone SP, Sader HS. In vitro activity of lefamulin against contemporary Staphylococcus aureus isolates from patients in Europe (sentry 2016) [abstract no P1823 plus poster]. In: European congress of clinical microbiology and infectious diseases. 21–24 April 2018; Madrid.
Zhanel GG, Ennis K, Vercaigne L, Walkty A, Gin AS, Embil J, et al. A critical review of the fluoroquinolones: focus on respiratory tract infections. Drugs. 2002;62(1):13–59.
File TM Jr, Goldberg L, Paukner S, Das A, Gelone SP, Saviski J, et al. Efficacy of lefamulin versus moxifloxacin against common pathogens in adults with community-acquired bacterial pneumonia (CABP): results from the phase 3 lefamulin evaluation against pneumonia (LEAP 1) study [abstract no 2386 plus poster]. IDWeek 2018. 3–7 October 2018; San Francisco.
Paukner S, Flamm RK, Gelone SP, Sader HS. In vitro activity of lefamulin (lef) against bacterial pathogens causing community-acquired bacterial pneumonia (CABP): SENTRY surveillance 2016 results from Asia-Pacific (APAC) and Latin America (LA) [abstract no 1365 plus poster]. IDWeek 2018. 3–7 October 2018; San Francisco.
Paukner S, Flamm RK, Gelone SP, Sader HS. In vitro activity of lefamulin (lef) against bacterial pathogens commonly causing community-acquired bacterial pneumonia (CABP): 2016 SENTRY data from the United States [abstract no 1353 plus poster]. IDWeek 2018. 3–7 October 2018; San Francisco.
Paukner S, Ryan Arends SJ, Gelone SP, Sader HS. In vitro activity of lefamulin against bacterial pathogens causing community-acquired bacterial pneumonia: SENTRY surveillance 2017–2018 results from the United States [abstract no 703 plus poster]. IDWeek 2019. 2–6 October 2019; Washington.
Adam HJ, Baxter MR, Golden A, Lagace-Wiens PR, Walkty A, Karlowsky JA, et al. Activity of lefamulin versus Streptococcus pneumoniae respiratory and blood isolates: CANWARD surveillance study 2015-2018 [abstract no 71 plus poster]. AMMI 2020.
Zhanel GG, Dueck M, Hoban DJ, Vercaigne LM, Embil JM, Gin AS, et al. Review of macrolides and ketolides focus on respiratory tract infections. Drugs. 2001;61(4):443–98.
Sader HS, Paukner S, Ryan Arends SJ, Gelone SP, Mendes RE. Antimicrobial activity of lefamulin against a large longitudinal collection of clinical bacterial isolates collected worldwide: results from the SENTRY antimicrobial surveillance programme [abstract no P1823 plus poster]. In: European congress of clinical microbiology and infectious diseases. 13–16 April 2019; Amsterdam.
Paukner S, Gelone SP, Sader HS. In vitro activity of lefamulin against isolates commonly causing community-acquired bacterial pneumonia collected during the SENTRY surveillance programme 2017 in Europe [abstract no P1828 plus poster]. In: European congress of clinical microbiology and infectious diseases. 13–16 April 2019; Amsterdam.
Paukner S, Flamm RK, Schuchert J, Gelone SP, Sader HS. In vitro activity of lefamulin against S. aureus collected worldwide from hospitalized patients with bacterial pneumonia [abstract no 1218 plus poster]. IDWeek 2017. 4–8 October 2017; San Diego.
Paukner S, Sader HS, Streit JM, Flamm RK, Gelone SP. In vitro activity of lefamulin against a global collection of bacterial pathogens commonly causing community-acquired bacterial pneumonia (CABP) SENTRY 2015 [abstract no 1220 plus poster]. IDWeek 2017. 4–8 October 2017; San Diego.
Paukner S, Sader HS, Ivezic-Schoenfeld Z, Jones RN. Antimicrobial activity of the pleuromutilin antibiotic BC-3781 against bacterial pathogens isolated in the SENTRY antimicrobial surveillance program in 2010. Antimicrob Agents Chemother. 2013;57(9):4489–95.
Sader HS, Paukner S, Ivezic-schoenfeld Z, Biedenbach DJ, Schmitz FJ, Jones RN. Antimicrobial activity of the novel pleuromutilin antibiotic BC-3781 against organisms responsible for community-acquired respiratory tract infections (CARTIs). J Antimicrob Chemother. 2012;67(5):1170–5.
Paukner SN. Nabriva Therapeutics. Personal communication. June 10, 2020.
Waites KB, Crabb DM, Duffy LB, Jensen JS, Liu Y, Paukner S. In vitro activities of lefamulin and other antimicrobial agents against macrolide-susceptible and macrolide-resistant Mycoplasma pneumoniae from the United States, Europe, and China. Antimicrob Agent Chemother. 2017;61(2):e02008-e2016.
Jensen JS, Paukner S. Lefamulin is highly active in vitro against multi-drug resistant Mycoplasma genitalium strains [abstract no P1335 plus poster]. In: European congress of clinical microbiology and infectious diseases. 22–25 April 2017; Vienna.
Paukner S, Gruss A, Jensen JS. In vitro activity of lefamulin against sexually transmitted bacterial pathogens. Antimicrob Agents Chemother. 2018;62(5):e02380-e2417.
Bradshaw CS, Jensen JS, Waites KB. New horizons in mycoplasma genitalium treatment. J Infect Dis. 2017;216(Suppl 2):S412–9.
Jacobsson S, Paukner S, Golparian D, Jensen JS, Unemo M. In vitro activity of the novel pleuromutilin lefamulin (BC-2781) and effect of efflux pump inactivation on multidrug-resistant and extensively drug-resistant Neisseria gonorrhoeae. Antimicrob Agents Chemother. 2017;61(11):e01497-e1517.
Wicha WW, Prince WT, Lell C, Heilmayer W, Gelone SP. Pharmacokinetics and tolerability of lefamulin following intravenous and oral dosing. J Antimicrob Chemother. 2019;74(Suppl 3):19–26.
Zhang L, Wicha WW, Bhavnani SM, Rubino CM. Prediction of lefamulin epithelial lining fluid penetration after intravenous and oral administration using phase 1 data and population pharmacokinetics methods. J Antimicrob Chemother. 2019;74(Suppl 3):27–34.
HIGHLIGHTS OF PRESCRIBING INFORMATION. These highlights do not include all the information needed to use XENLETA TM safely and effectively. See full prescribing information for XENLETA. XENLETA (lefamulin) for injection, for intravenous use XENLETA (lefam [Internet]). www.fda.gov/medwatch. Accessed 20 May 2020.
Zeitlinger M, Schwameis R, Burian A, Burian B, Matzneller P, Müller M, et al. Simultaneous assessment of the pharmacokinetics of a pleuromutilin, lefamulin, in plasma, soft tissues and pulmonary epithelial lining fluid. J Antimicrob Chemother. 2016;71(4):1022–6.
Wicha WW, Lell C, Obermayr F, Logan DK, Prince WT. An age and gender study investigating the safety, tolerance and pharmacokinetics of BC-3781 [abstract no A1-019 plus poster]. In: Interscience conference on antimicrobial agents and chemotherapy. 12–15 September 2010; Boston.
Schmidt U, Wicha WW, Obermayr F, Novak R, Prince W. BC-3781: evaluation of the CYP3a interaction potential [abstract no P1524 plus poster]. In: European congress of clinical microbiology and infecious diseases. 2011; Vienna.
Rubino CM, Xue B, Bhavnani SM, Prince WT, Ivezic-Schoenfeld Z, Wicha WW, et al. Population pharmacokinetic analyses for BC-3781 using phase 2 data from patients with acute bacterial skin and skin structure infections. Antimicrob Agents Chemother. 2015;59(1):282–8.
Wicha WW, Craig WA, Andes D. In vivo pharmacodynamics of lefamulin, the first systemic pleuromutilin for human use, in a neutropenic murine thigh infection model. J Antimicrob Chemother. 2019;74(Suppl 3):5–10.
Wicha WW, Marbury TC, Dowell JA, Lykens L, Leister C, Ermer J, et al. Pharmacokinetics and safety of lefamulin after single intravenous dose administration in subjects with impaired hepatic function [abstract no 722 plus poster]. IDWeek 2019. 2–6 October 2019; Washington.
Wicha WW, Marbury TC, Dowell JA, Lykens L, Leister C, Ermer J, et al. Pharmacokinetics and safety of lefamulin after single intravenous dose administration in subjects with impaired renal function and in those requiring hemodialysis [abstract no 705 plus poster]. IDWeek 2019. 2–6 October 2019; Washington.
Onufrak NJ, Ganesan H, Wicha WW, Gelone SP, Bhavnani SM, Rubino CM. Population pharmacokinetic analysis for lefamulin using data from healthy volunteers and infected patients [abstract no P1925 plus poster]. In: European congress of clinical microbiology and infectious diseases. 13–16 April 2019; Amsterdam.
Wicha WW, Strickmann DB, Paukner S. Pharmacokinetics/pharmacodynamics of lefamulin in a neutropenic murine pneumonia model with Staphylococcus aureus and Streptococcus pneumoniae. J Antimicrob Chemother. 2019;74(Suppl 3):11–8.
Bhavnani SM, Zhang L, Hammel JP, Rubino CM, Bader JC, Sader HS, et al. Pharmacokinetic/pharmacodynamic target attainment analyses to support intravenous and oral lefamulin dose selection for the treatment of patients with community-acquired bacterial pneumonia. J Antimicrob Chemother. 2019;74(Suppl 3):35–41.
Wicha WW, Kappes CB, Fischer E. Efficacy of lefamulin against Staphylococcus aureus-induced bacteremia in a neutropenic and immunocompetent murine model [abstract no 1509 plus poster]. IDWeek 2017. 4–8 October 2017; San Diego.
Wicha WW, Paukner S, Strickland DB, Bhavnani SM AP. Pharmacokinetics-pharmacodynamics of lefamulin in a neutropenic murine lung infection model [abstract no A-037 plus poster]. In: Interscience conference on antimicrobial agents and chemotherapy. September 2015; San Diego.
Prince WT, Ivezic-Schoenfeld Z, Lell C, Tack KJ, Novak R, Obermayr F, et al. Phase II clinical study of BC-3781, a pleuromutilin antibiotic, in treatment of patients with acute bacterial skin and skin structure infections. Antimicrob Agents Chemother. 2013;57(5):2087–94.
File TM, Goldberg L, Das A, Sweeney C, Saviski J, Gelone SP, et al. Efficacy and safety of intravenous-to-oral lefamulin, a pleuromutilin antibiotic, for the treatment of community-acquired bacterial pneumonia: the phase III lefamulin evaluation against pneumonia (LEAP 1) trial. Clin Infect Dis. 2019;69(11):1856–67.
Alexander E, Goldberg L, Das AF, Moran GJ, Sandrock C, Gasink LB, et al. Oral lefamulin vs moxifloxacin for early clinical response among adults with community-acquired bacterial pneumonia: the LEAP 2 randomized clinical trial. JAMA. 2019;322(17):1661–71.
Aschenbrenner D. New antibiotic for community-acquired bacterial pneumonia. Am J Nurs. 2019;119:20–1.
Lexicomp Online. Interaction Monograph, Lefamulin/Ketoconazole: Wolters Kluwer Clinical Drug Information, Inc.; 2019. https://www.online.lexi.com. Accessed 11 July 2020.
Lexicomp Online. Interaction Monograph, Lefamulin/P-gp Inducers and P-gp Inhibitors: Wolters Kluwer Clinical Drug Information, Inc.; 2019. https://www.online.lexi.com. Accessed 11 July 2020.
Lexicomp Online. Interaction Monograph, Lefamulin/QT-prolonging CYP3A4 Substrates: Wolters Kluwer Clinical Drug Information, Inc.; 2019. https://www.online.lexi.com. Accessed 12 July, 2020.
Lexicomp Online. Interaction Monograph, Lefamulin/Apixaban: Wolters Kluwer Clinical Drug Information, Inc.; 2019. https://www.online.lexi.com. Accessed 12 July 2020.
Lexicomp Online. Interaction Monograph, Lefamulin/Domperidone [Internet]: Wolters Kluwer Clinical Drug Information, Inc.; 2019. https://www.online.lexi.com. Accessed 12 July 2020.
Lexicomp Online. Interaction Monograph, Lefamulin/Sirolimus [Internet]: Wolters Kluwer Clinical Drug Information, Inc.; 2019. https://www.online.lexi.com. Accessed 12 July 2020.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Zhanel has received research grants from Sunovion Pharmaceuticals and Nabriva Therapeutics. No conflicts are declared for Christina Deng, Sheryl Zelenitsky, Courtney K. Lawrence, Heather J. Adam, Alyssa Golden, Rachel Hink, Liam Berry, Frank Schweizer, Michael A. Zhanel, Denice Bay, Philippe Lagacé-Wiens, Andrew Walkty, Lionel Mandell, Joseph P. Lynch III and James A. Karlowsky. Dr. Irfan is part of the Sunovion speaker’s bureau.
Funding statement
The authors are grateful to Sunovion Pharmaceuticals and Nabriva Therapeutics for their assistance with literature retrieval and an unrestricted research grant to aid in funding Christina Deng.
Contribution of authors
George G. Zhanel (involved in design and concept of entire manuscript and drafting all sections, senior and corresponding author), Christina Deng (involved in design and concept of entire manuscript and drafting all sections), Sheryl Zelenitsky (involved in design and concept of entire manuscript and drafting pharmacokinetic and pharmacodynamics sections), Courtney K. Lawrence, (involved in design and concept of entire manuscript and drafting pharmacokinetic and pharmacodynamics sections), Heather J. Adam (involved in design and concept of entire manuscript and drafting microbiology section), Alyssa Golden (involved in design and concept of entire manuscript and drafting mechanisms of action and resistance sections), Liam Berry (involved in design and concept of entire manuscript and drafting chemistry and microbiology sections), Frank Schweizer (involved in design and concept of entire manuscript and drafting chemistry and microbiology sections), Michael A. Zhanel (involved in design and concept of entire manuscript and drafting introduction, mechanisms of action/resistance, chemistry and microbiology sections), Neal Irfan (involved in design and concept of entire manuscript and drafting adverse effects, drug interactions and place in therapy sections), Denice Bay (involved in design and concept of entire manuscript and drafting introduction, mechanisms of action/resistance sections), Philippe Lagacé-Wiens (involved in design and concept of entire manuscript and drafting animal models section), Andrew Walkty (involved in design and concept of entire manuscript and drafting clinical trials, adverse effects and place in therapy sections), Lionel Mandell (involved in design and concept of entire manuscript and drafting clinical trials section), Joseph P. Lynch III (involved in design and concept of entire manuscript and drafting clinical trials and place in therapy sections), and James A. Karlowsky (involved in design and concept of entire manuscript and drafting all sections).
Rights and permissions
About this article
Cite this article
Zhanel, G.G., Deng, C., Zelenitsky, S. et al. Lefamulin: A Novel Oral and Intravenous Pleuromutilin for the Treatment of Community-Acquired Bacterial Pneumonia. Drugs 81, 233–256 (2021). https://doi.org/10.1007/s40265-020-01443-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-020-01443-4