Abstract
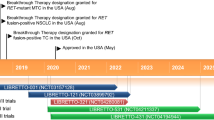
Pralsetinib (GAVRETO™, Blueprint Medicines Corporation) is a selective rearranged during transfection (RET) inhibitor being developed for the treatment of various solid tumours. RET is a well described proto-oncogene present in multiple cancers including non-small cell lung cancer (NSCLC), papillary thyroid cancer, and medullary thyroid carcinoma (MTC). Pralsetinib was recently granted accelerated approval for the treatment of metastatic RET fusion-positive NSCLC in the USA and is under regulatory review in the USA for RET fusion-positive thyroid cancer and RET mutation-positive MTC; pralsetinib is under regulatory review in the EU for RET fusion-positive NSCLC. This article summarizes the milestones in the development of pralsetinib leading to this first approval.
Similar content being viewed by others
References
Zhao Z, Fu T, Gao J, et al. Identifying novel oncogenic RET mutations and characterising their sensitivity to RET-specific inhibitors. J Med Genet. 2020;13:13.
Blueprint Medicines. Blueprint Medicines announces FDA approval of GAVRETO™ (pralsetinib) for the treatment of adults with metastatic RET fusion-positive non-small cell lung cancer [media release]. 4 Sept 2020 https://www.blueprintmedicines.com.
US Food and Drug Administration. GAVRETO™ (pralsetinib) capsules, for oral use: US prescribing information. 2020. https://www.blueprintmedicines.com/uspi/GAVRETO.pdf. Accessed 18 Sept 2020.
Blueprint Medicines, CStone Pharmaceuticals. Blueprint Medicines and CStone Pharmaceuticals announce exclusive collaboration and license agreement to develop and commercialize avapritinib, BLU-554 and BLU-667 in greater China [media release]. 4 June 2018 https://www.blueprintmedicines.com.
Blueprint Medicines. Blueprint Medicines announces global collaboration with Roche to develop and commercialize pralsetinib for patients with RET-altered cancers [media release]. 13 July 2020 https://www.blueprintmedicines.com.
Rahal R, Maynard M, Hu W, et al. BLU-667 is a potent and highly selective RET inhibitor being developed for RET-driven cancers [abstract no. B151]. Mol Cancer Ther. 2017;17(1 Suppl 1).
El Osta B, Ramalingham SS. RET fusion: joining the ranks of targetable molecular drivers in NSCLC. JTO Clin Res Rep. 2020;1(3):1–11.
US Food and Drug Administration. NDA multidisciplinary review and evaluation (213721): pralsetinib. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/213721Orig1s000MultidisciplineR.pdf. Accessed 9 Oct 2020.
Hu M, Subbiah V, Wirth LJ, et al. Results from the registrational phase I/II ARROW trial of pralsetinib (BLU-667) in patients (pts) with advanced RET mutation-positive medullary thyroid cancer (RET+ MTC) [abstract no. 1913O]. Ann Oncol. 2020;31(Suppl 4):S1084.
Subbiah V, Hu M-N, Gainor JF, et al. Clinical activity of the RET inhibitor pralsetinib (BLU-667) in patients with RET fusion + solid tumors [abstract no. 109]. In: 56th Annual Meeting of the American Society of Clinical Oncology. 2020.
Thermo Fischer Scientific Inc. FDA approves first NGS-based companion diagnostic for RET fusion-positive non-small cell lung cancer [media release]. 8 Sept 2020 https://thermofisher.mediaroom.com.
Besse B, Felip E, Clifford C, et al. AcceleRET Lung: a phase III study of first-line pralsetinib in patients (pts) with RET-fusion + advanced/metastatic non-small cell lung cancer (NSCLC) [abstract no. TPS9633]. In: 56th Annual Meeting of the American Society of Clinical Oncology. 2020.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. A. Markham is a contracted employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
Enhanced material for this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshare.13082027.
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Markham, A. Pralsetinib: First Approval. Drugs 80, 1865–1870 (2020). https://doi.org/10.1007/s40265-020-01427-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-020-01427-4