Abstract
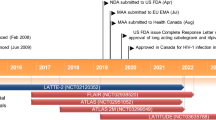
Fostemsavir (Rukobia), a prodrug of the HIV-1 attachment inhibitor temsavir, is a first-in-class treatment for HIV infection being developed by ViiV Healthcare. Based on the results of the phase III BRIGHTE trial fostemsavir was recently approved in the USA for the treatment of patients with HIV not able to be treated with other therapies. This article summarizes the milestones in the development of fostemsavir leading to this first approval.
Similar content being viewed by others
Change history
09 September 2020
The original article can be found online
References
US Food & Drug Administration. FDA approves new HIV treatment for patients with limited treatment options [media release]. 2 July 2020. https://www.fda.gov.
US Food & Drug Administration. Rukobia (fostemsavir): US prescribing information. 2020. https://www.accessdata.fda.gov. Accessed 31 July 2020.
ViiV Healthcare. ViiV Healthcare submits regulatory application to the European Medicines Agency for fostemsavir, an investigational, first-in-class attachment inhibitor for the treatment of HIV in adults with few treatment options available [media release]. 10 Jan 2020. https://www.viivhealthcare.com.
Bristol-Myers Squibb. Bristol-Myers Squibb completes previously announced sale of its HIV R portfolio to ViiV Healthcare [media release]. 2016. https://www.bms.com.
Pancera M, Lai YT, Bylund T, et al. Crystal structures of trimeric HIV envelope with entry inhibitors BMS-378806 and BMS-626529. Nat Chem Biol. 2017;13(10):1115–22.
Nowicka-Sans B, Gong YF, McAuliffe B, et al. In vitro antiviral characteristics of HIV-1 attachment inhibitor BMS-626529, the active component of the prodrug BMS-663068. Antimicrob Agents Chemother. 2012;56(7):3498–507.
Zhou N, Nowicka-Sans B, McAuliffe B, et al. Genotypic correlates of susceptibility to HIV-1 attachment inhibitor BMS-626529, the active agent of the prodrug BMS-663068. J Antimicrob Chemother. 2014;69(3):573–81.
Lataillade M, Zhou N, Joshi SR, et al. Viral drug resistance through 48 weeks, in a phase 2b, randomized, controlled trial of the HIV-1 attachment inhibitor prodrug, fostemsavir. J Acquir Immune Defic Syndr JAIDS. 2018;77(3):299–307.
Nettles RE, Schurmann D, Zhu L, et al. Pharmacodynamics, safety, and pharmacokinetics of BMS-663068, an oral HIV-1 attachment inhibitor in HIV-1-infected subjects. J Infect Dis. 2012;206(7):1002–111.
Li Z, Zhou N, Sun Y, et al. Activity of the HIV-1 attachment inhibitor BMS-626529, the active component of the prodrug BMS-663068, against CD4-independent viruses and HIV-1 envelopes resistant to other entry inhibitors. Antimicrob Agents Chemother. 2013;57(9):4172–80.
Gartland M, Ackerman P, Mannino F, et al. Clinical significance of gp120 polymorphisms, TMR IC50 FC and HIV-1 subtype in BRIGHTE [abstract no. 503]. In: 27th conference on retroviruses and opportunistic infections. 2020.
Lagishetty C, Moore K, Ackerman P, et al. Effects of temsavir, active moiety of antiretroviral agent fostemsavir, on QT interval: results from a phase I study and an exposure-response analysis. Clin Transl Sci. 2020;13:769–76.
Sevinsky H, Magee M, Ackerman P, et al. Pharmacokinetics of temsavir, the active moiety of the prodrug fostemsavir, in subjects with hepatic impairment [abstract no. 1390]. Open Forum Infect Dis. 2017;4(Suppl 1):S430.
Moore K, Magee M, Gunshenan M, et al. Impact of mild, moderate and severe renal impairment and hemodialysis on temsavir pharmacokinetics following oral administration of fostemsavir, an attachment inhibitor for heavily treatment-experienced HIV-1 infected patients [abstract no. P261]. J Int AIDS Soc. 2018;21(Suppl 8):169.
Magee M, Sevinsky H, Ackerman P, et al. The effect of fostemsavir on the pharmacokinetics of a combined oral contraceptive (OC) containing ethinyl estradiol (EE) and norethindrone (NE) in healthy female subjects [abstract no. MOPEB0339]. In: 9th international AIDS society conference on HIV science. 2017.
Moore KP, Mageau AS, Magee M, et al. Fostemsavir drug-drug interaction profile, an attachment inhibitor and oral prodrug of temsavir, for heavily treatment experienced HIV-1-infected patients [abstract no. 2500]. Open Forum Infect Dis. 2019;6(Suppl 2):S867.
Moore K, Magee M, Sevinsky H, et al. Methadone and buprenorphine pharmacokinetics and pharmacodynamics when coadministered with fostemsavir to opioid-dependent, human immunodeficiency virus seronegative participants. Br J Clin Pharmacol. 2019;85(8):1771–800.
Zhu L, Hruska M, Hwang C, et al. Pharmacokinetic interactions between BMS-626529, the active moiety of the HIV-1 attachment inhibitor prodrug BMS-663068, and ritonavir or ritonavir-boosted atazanavir in healthy subjects. Antimicrob Agents Chemother. 2015;59(7):3816–22.
Kozal M, Aberg J, Pialoux G, et al. Fostemsavir in adults with multidrug-resistant HIV-1 infection. N Engl J Med. 2020;382(13):1232–43.
Lataillade M, Lalezari J, Aberg J, et al. Week 96 safety and efficacy of the novel HIV-1 attachment inhibitor prodrug fostemsavir in heavily treatment-experienced participants infected with multi-drug resistant HIV-1 (BRIGHTE study) [abstract no. MOAB0102]. J Int AIDS Soc. 2019;22(Suppl 5):15.
Thompson M, Urbina FM, Latiff G, et al. Long-term safety efficacy of fostemsavir in treatment-experienced HIV participants [abstract no. 483]. In: 26th conference on retroviruses and opportunistic infections. 2019.
Lalezari JP, Latiff GH, Brinson C, et al. Safety and efficacy of the HIV-1 attachment inhibitor prodrug BMS-663068 in treatment-experienced individuals: 24 week results of AI438011, a phase 2b, randomised controlled trial. Lancet HIV. 2015;2(10):e427–e437437.
Acknowledgements
During the peer review process, the manufacturer of fostemsavir was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
A. Markham is a contracted employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
The original article has been update: Due to Acknowledgement update.
Enhanced material for this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshare.12792599.
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Markham, A. Fostemsavir: First Approval. Drugs 80, 1485–1490 (2020). https://doi.org/10.1007/s40265-020-01386-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-020-01386-w