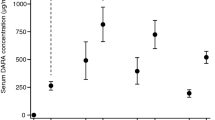
Abstract
Intravenous daratumumab (DARZALEX®), a human monoclonal antibody targeting CD38, is approved in the EU and USA for use in combination with bortezomib, thalidomide and dexamethasone for the treatment of adults with newly diagnosed multiple myeloma (MM) who are eligible for autologous stem cell transplantation. A subcutaneous formulation of daratumumab has also been approved in the EU and USA (DARZALEX FASPRO™) for use in MM. In the pivotal phase III CASSIOPEIA trial in adults with newly diagnosed, transplant-eligible MM, the addition of intravenous daratumumab to bortezomib, thalidomide and dexamethasone significantly increased the proportion of patients with a stringent complete response and significantly prolonged progression-free survival; overall survival data are not yet mature. Some facets of health-related quality of life were improved by the addition of daratumumab. The addition of daratumumab had a minimal effect on overall toxicity and the most common grade ≥ 3 adverse events with daratumumab combination therapy were haematological (e.g. neutropenia, lymphopenia). The approval of daratumumab as combination therapy in patients with newly diagnosed, transplant-eligible MM expands the range of MM treatment settings in which daratumumab is an option and the availability of the subcutaneous formulation will likely be of benefit to patients.

adapted from McKeage and Lyseng-Williamson


Similar content being viewed by others
Change history
17 December 2020
Correction in reviewer name.
References
Al Hamed R, Bazarbachi AH, Malard F, et al. Current status of autologous stem cell transplantation for multiple myeloma. Blood Cancer J. 2019;9:44.
Fonseca R, Abouzaid S, Bonafede M, et al. Trends in overall survival and costs of multiple myeloma, 2000–2014. Leukemia. 2017;31(9):1915–21.
Mahajan S, Tandon N, Kumar S. The evolution of stem-cell transplantation in multiple myeloma. Ther Adv Hematol. 2018;9(5):123–33.
Moreau P, San Miguel J, Sonneveld P, et al. Multiple myeloma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(Suppl 4):52–61.
van de Donk N, Richardson PG, Malavasi F. CD38 antibodies in multiple myeloma: back to the future. Blood. 2018;131(1):13–29.
Krejcik J, Casneuf T, Nijhof IS, et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016;128(3):384–94.
Janssen-Cilag International NV. DARZALEX (daratumumab): EU summary of product charateristics. 2020. https://ec.europa.eu/. Accessed 3 Aug 2020.
Janssen Biotech. DARZALEX (daratumumab): US prescribing information 2020. https://www.accessdata.fda.gov/. Accessed 3 Aug 2020.
Blair HA. Daratumumab: a review in relapsed and/or refractory multiple myeloma. Drugs. 2017;77(18):2013–24.
Syed YY. Daratumumab: a review in combination therapy for transplant-ineligible newly diagnosed multiple myeloma. Drugs. 2019;79(4):447–54.
Janssen Biotech. DARZALEX FASPRO™: US prescribing information. 2020. https://www.accessdata.fda.gov/. Accessed 3 Aug 2020.
McKeage K, Lyseng-Williamson KA. Daratumumab in multiple myeloma: a guide to its use as monotherapy in the EU. Drugs Ther Perspect. 2016;32:463–9.
Adams HC, Stevenaert F, Krejcik J, et al. High-parameter mass cytometry evaluation of relapsed/refractory multiple myeloma patients treated with daratumumab demonstrates immune modulation as a novel mechanism of action. Cytometry A. 2019;95(3):279–89.
de Weers M, Tai YT, van der Veer MS, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186(3):1840–8.
Overdijk MB, Jansen JH, Nederend M, et al. The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via Fcγ receptor-mediated cross-linking. J Immunol. 2016;197(3):807–13.
Overdijk MB, Verploegen S, Bogels M, et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. mAbs. 2015;7(2):311–21.
Kitadate A, Kobayashi H, Abe Y, et al. Pre-treatment CD38-positive regulatory T cells affect the durable response to daratumumab in relapsed/refractory multiple myeloma patients. Haematologica. 2020;105:e37–40.
Casneuf T, Xu XS, Adams HC 3rd, et al. Effects of daratumumab on natural killer cells and impact on clinical outcomes in relapsed or refractory multiple myeloma. Blood Adv. 2017;1(23):2105–14.
Nijhof IS, Casneuf T, van Velzen J, et al. CD38 expression and complement inhibitors affect response and resistance to daratumumab therapy in myeloma. Blood. 2016;128(7):959–70.
Nijhof IS, Groen RW, Lokhorst HM, et al. Upregulation of CD38 expression on multiple myeloma cells by all-trans retinoic acid improves the efficacy of daratumumab. Leukemia. 2015;29(10):2039–49.
Plesner T, Krejcik J. Daratumumab for the treatment of multiple myeloma. Front Immunol. 2018;9:1228.
Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(8):754–66.
Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(14):1319–31.
Chapuy CI, Nicholson RT, Aguad MD, et al. Resolving the daratumumab interference with blood compatibility testing. Transfusion (Paris). 2015;55(6 Pt 2):1545–54.
McCudden C, Axel AE, Slaets D, et al. Monitoring multiple myeloma patients treated with daratumumab: teasing out monoclonal antibody interference. Clin Chem Lab Med. 2016;54(6):1095–104.
Xu XS, Dimopoulos MA, Sonneveld P, et al. Pharmacokinetics and exposure-response analyses of daratumumab in combination therapy regimens for patients with multiple myeloma. Adv Ther. 2018;35(11):1859–72.
Clemens PL, Yan X, Lokhorst HM, et al. Pharmacokinetics of daratumumab following intravenous infusion in relapsed or refractory multiple myeloma after prior proteasome inhibitor and immunomodulatory drug treatment. Clin Pharmacokinet. 2017;56(8):915–24.
Xu XS, Yan X, Puchalski T, et al. Clinical implications of complex pharmacokinetics for daratumumab dose regimen in patients with relapsed/refractory multiple myeloma. Clin Pharmacol Ther. 2017;101(6):721–4.
Mateos MV, Nahi H, Legiec W, et al. Subcutaneous versus intravenous daratumumab in patients with relapsed or refractory multiple myeloma (COLUMBA): a multicentre, open-label, non-inferiority, randomized, phase 3 trial. Lancet Haematol. 2020;7(5):e370–e380380.
Moreau P, Attal M, Hulin C, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet. 2019;394(10192):29–38.
Hulin C, Moreau P, Attal M, et al. Stem cell yield and transplantation in transplant-eligible newly diagnosed multiple myeloma patients receiving daratumumab + bortezomib/thalidomide/ dexamethasone (D-VTd): phase 3 CASSIOPEIA study [abstract no. PF598 and poster]. HemaSphere. 2019;3(Suppl 1):252.
Sonneveld P, Attal M, Perrot A, et al. Daratumumab plus bortezomib, thalidomide, and dexamethasone (D-VTd) in transplant-eligible newly diagnosed multiple myeloma (NDMM): subgroup analysis of high-risk patients (pts) in CASSIOPEIA [abstract no. OAB-003]. Clin Lymphoma Myeloma Leuk. 2019;19(Suppl 10):e2–e3.
Avet-Loiseau H, Moreau P, van der Velden VHJ, et al. Efficacy of daratumumab + bortezomib/thalidomide/dexamethasone (D-VTd) in transplant-eligible newly diagnosed multiple myeloma based on minimal residual disease status: analysis of CASSIOPEIA [abstract no. S874]. HemaSphere. 2019;3(Suppl 1):391–2.
Moreau P, Zweegman S, Perrot A, et al. Evaluation of the prognostic value of positron emission tomography-computed tomography (PET-CT) at diagnosis and follow-up in transplant-eligible newly diagnosed multiple myeloma (TE NDMM) patients treated in the phase 3 CASSIOPEIA study: results of the CASSIOPET companion study [abstract]. Blood. 2019;134(Suppl 1).
Roussel M, Moreau P, Attal M, et al. Improvement in health-related quality of life for newly diagnosed multiple myeloma transplant-eligible patients treated with daratumumab, bortezomib, thalidomide, and dexamethasone: CASSIOPEIA study [abstract no. PS1377 and poster]. HemaSphere. 2019;3(Suppl 1):630.
Chari A, San-Miguel J, McCarthy H, et al. Subcutaneous daratumumab plus standard treatment regimens in patients with multiple myeloma across lines of therapy: PLEIADES study update. Blood. 2019;134(Suppl 1):3152.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN Guidelines): multiple myeloma (version 4.2020). https://www.nccn.org. Accessed 3 Aug 2020.
Perrot A, Lauwers-Cances V, Corre J, et al. Minimal residual disease negativity using deep sequencing is a major prognostic factor in multiple myeloma. Blood. 2018;132(23):2456–64.
Richardson PG, Delforge M, Beksac M, et al. Management of treatment-emergent peripheral neuropathy in multiple myeloma. Leukemia. 2012;26:595–608.
Moreau P, Attal M, Facon T, et al. A matching-adjusted indirect comparison (MAIC) of bortezomib-thalidomide-dexamethasone (VTd) and daratumumab plus VTd (D-VTd) versus bortezomib-dexamethasone (Vd) in patients with newly diagnosed multiple myeloma (NDMM) who are transplant eligible (TE) [abstract no. SP-008]. Clin Lymphoma Myeloma Leuk. 2019;19(Suppl 10):e200-e1.
Moreau P, Attal M, Facon T, et al. A matching-adjusted indirect comparison (MAIC) of daratumumab-bortezomib-thalidomide-dexamethasone (D-VTd) versus bortezomib-lenalidomide-dexamethasone (VRd) in patients (pts) with newly diagnosed multiple myeloma (NDMM) who are transplant eligible [abstract no. SP-007]. Clin Lymphoma Myeloma Leuk. 2019;19(Suppl 10):e199-e200.
Sonneveld P, Dejoie T, Zweegman S, et al. A matching-adjusted indirect comparison (MAIC) of bortezomib-thalidomide-dexamethasone (VTd) and daratumumab plus VTd (D-VTd) versus bortezomib-cyclophosphamide-dexamethasone (VCd) in patients (pts) with newly diagnosed multiple myeloma (NDMM) who are transplant eligible (TE) [abstract no. SP-009]. Clin Lymphoma Myeloma Leuk. 2019;19(Suppl 10):e201-e2.
Voorhees PM, Kaufman JL, Laubach JP, et al. Daratumumab, lenalidomide, bortezomib & dexamethasone for transplant-eligible newly diagnosed multiple myeloma: GRIFFIN. Blood. 2020. https://doi.org/10.1182/blood.2020005288.
Rajkumar SV, Harousseau JL. Next-generation multiple myeloma treatment: a pharmacoeconomic perspective. Blood. 2016;128(24):2757–64.
Anderson KC, Landgren O, Arend RC, et al. Humanistic and economic impact of subcutaneous versus intravenous administration of oncology biologics. Future Oncol. 2019;15(28):3267–81.
Acknowledgements
During the peer review process, the manufacturer of daratumumab was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
Yvette N. Lamb is a salaried employee of Adis International Ltd/Springer Nature, is responsible for the article content and declares no relevant conflicts of interest.
Ethics approval, Consent to participate and consent for publication, Availability of data and material, Code availability
Not applicable.
Additional information
Enhanced material for this Adis Drug Evaluation can be found at https://doi.org/10.6084/m9.figshare.12678944.
The manuscript was reviewed by: N. Callander, University of Wisconsin Carbone Cancer Center, Madison, WI, USA; C. Cerchione, Hematology Unit, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS, Meldola, Italy; S. Knop, Medizinische Klinik und Poliklinik II, Universitätsklinikum Würzburg, Würzburg, Germany; S. J. Kumar, Hematology and Internal Medicine, Mayo Clinic, Rochester, MN, USA.
Rights and permissions
About this article
Cite this article
Lamb, Y.N. Daratumumab: A Review in Combination Therapy for Transplant-Eligible Newly Diagnosed Multiple Myeloma. Drugs 80, 1455–1464 (2020). https://doi.org/10.1007/s40265-020-01385-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-020-01385-x