Abstract
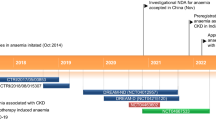
Vadadustat (VAFSEO®) is a prolyl hydroxylase inhibitor being developed by Akebia Therapeutics, Inc. (Akebia) for the treatment of anaemia associated with chronic kidney disease (CKD). Akebia is collaborating with Mitsubishi Tanabe Pharma Corporation on the development and commercialization of vadadustat in Japan and with Otsuka Pharmaceutical Co. Ltd on the development and commercialization of vadadustat in the USA, the EU and certain other territories. The drug is approved in Japan for use in adult patients with anaemia associated with CKD and regulatory submissions are planned in the USA and the EU. This article summarizes the milestones in the development of vadadustat leading to this first approval.
Similar content being viewed by others
References
Akebia Therapeutics Inc. Akebia and Mitsubishi Tanabe Pharma announce collaboration to develop and commercialize vadadustat in Asia [media release]. 14 Dec 2015. https://akebia.com/.
Zuk A, Si Z, Loi S, et al. Preclinical characterization of vadadustat (AKB-6548), an oral small molecule hypoxia inducible factor prolyl-4-hydroxylase inhibitor, for the potential treatment of renal anemia [abstract no. SA-OR037]. J Am Soc Nephrol. 2019;30:90.
Sanghani NS, Haase VH. Hypoxia-inducible factor activators in renal anemia: current clinical experience. Adv Chronic Kidney Dis. 2019;26(4):253–66.
Mitsubishi Tanabe Pharma Corporation. Manufacturing and marketing approval of VAFSEO® tablets (HIF-PH inhibitor, vadadustat) for renal anemia in Japan [media release]. 29 Jun 2020. http://www.mt-pharma.co.jp.
Mitsubishi Tanabe Pharma Corporation. Vadadustat (VAFSEO tablets): Japanese prescribing information. 2020. https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/400315_39990D3F1020_1_01. Accessed 21 Jul 2020.
Akebia Therapeutics Inc. Akebia Therapeutics announces positive top-line results from global phase 3 program of vadadustat for treatment of anemia due to chronic kidney disease in adult patients on dialysis [media release]. 5 May 2020. https://akebia.com/.
Akebia Therapeutics Inc. Akebia closes $41 million Series C [media release]. 4 Jun 2013. https://akebia.com/.
Akebia Therapeutics Inc. Akebia and Otsuka Pharmaceutical announce collaboration to develop and commercialize vadadustat in the U.S. [media release]. 20 Dec 2016. https://akebia.com/.
Akebia Therapeutics Inc. Akebia and Otsuka expand relationship with collaboration to develop and commercialise vadadustat in Europe, China and other territories [media release]. 25 Apr 2017. https://akebia.com/.
Akebia Therapeutics Inc. Akebia and Vifor Pharma announce exclusive license agreement to provide vadadustat to Fresenius Medical Care in the U.S. upon FDA Approval [media release]. 15 May 2017. https://akebia.com/.
Akebia Therapeutics Inc. Akebia Therapeutics reports fourth quarter and full-year 2019 financial results and hosts conference call to discuss recent business highlights [media release]. 10 Mar 2020. https://akebia.com/.
Yeh TL, Leissing TM, Abboud MI, et al. Molecular and cellular mechanisms of HIF prolyl hydroxylase inhibitors in clinical trials. Chem Sci. 2017;8(11):7651–68.
US Securities and Exchange Commission. Akebia Therapeutics Form 10-K, March 2020. https://akebia.com/. Accessed 6 Aug 2020.
Sawant R, Paulson S, Burke L, et al. Evaluation of drug interaction with multiple doses of rabeprazole, a proton pump inhibitor, on the pharmacokinetics of vadadustat [abstract no. 328]. Am J Kidney Dis. 2020;75(4):631.
Chavan AB, Paulson SK, Burke L, et al. Effect of moderate hepatic impairment on pharmacokinetics of vadadustat, an oral hypoxia-inducible factor prolyl hydroxylase inhibitor (HIF-PHI) [abstract no. TH-PO369]. J Am Soc Nephrol. 2019;30:212.
Sawant R, Paulson S, Burke L, et al. Effect of food intake on the pharmacokinetics of vadadustat following single dose administration [abstract no. 327]. Am J Kidney Dis. 2020;75(4):631.
Nangaku M, Kondo K, Kokado Y, et al. Randomized, open-label, active-controlled (darbepoetin alfa), phase 3 study of vadadustat for treating anemia in non-dialysis-dependent CKD patients in Japan [abstract no. SA-PO229]. J Am Soc Nephrol. 2019;30:825.
Nangaku M, Kondo K, Ueta K, et al. Randomized, double-blinded, active-controlled (darbepoetin alfa), phase 3 study of vadadustat in CKD patients with anemia on hemodialysis in Japan [abstract no. TH-OR024]. J Am Soc Nephrol. 2019;30:8.
Nangaku M, Farag YMK, de Goma E, et al. Vadadustat, an oral hypoxia-inducible factor prolyl hydroxylase inhibitor, for treatment of anemia of chronic kidney disease: two randomized phase 2 trials in Japanese patients. Nephrol Dial Transplant. 2020. https://doi.org/10.1093/ndt/gfaa060.
Martin ER, Smith MT, Maroni BJ, et al. Clinical trial of vadadustat in patients with anemia secondary to stage 3 or 4 chronic kidney disease. Am J Nephrol. 2017;45(5):380–8.
Pergola PE, Spinowitz BS, Hartman CS, et al. Vadadustat, a novel oral HIF stabilizer, provides effective anemia treatment in nondialysis-dependent chronic kidney disease. Kidney Int. 2016;90(5):1115–22.
Haase VH, Chertow GM, Block GA, et al. Effects of vadadustat on hemoglobin concentrations in patients receiving hemodialysis previously treated with erythropoiesis-stimulating agents. Nephrol Dial Transplant. 2019;34(1):90–9.
Akebia Therapeutics Inc. Akebia initiates phase 3 PRO2TECT(TM) Program [media release]. 4 Jan 2016. https://akebia.com/.
Acknowledgements
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
A. Markham is a contracted employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
Enhanced material for this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshare.12782549.
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Markham, A. Vadadustat: First Approval. Drugs 80, 1365–1371 (2020). https://doi.org/10.1007/s40265-020-01383-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-020-01383-z