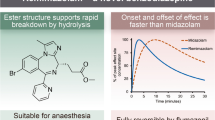
Abstract
Remimazolam (Anerem® in Japan; ByFavo™ in the USA; Aptimyda™ in the EU) is an ultra-short-acting intravenous (IV) benzodiazepine sedative/anesthetic being developed by PAION AG in conjunction with a number of commercial partners for use in anesthesia and procedural sedation. Remimazolam was approved on 23 January 2020 in Japan for use in general anesthesia in adult patients. Remimazolam is also undergoing regulatory assessment in South Korea for this indication and for use in procedural sedation in the USA, the EU and China. This article summarises the major milestones in the development of remimazolam for this first approval for the induction and maintenance of general anaesthesia, and its potential upcoming approvals in general anaesthesia and procedural sedation.
Similar content being viewed by others
References
Mundipharma Co. Ltd. Remimazolam besylate (Anerem): Japanese prescribing information. 2020. https://www.pmda.go.jp/. Accessed 2 Mar 2020.
Cosmo Pharmaceuticals. Cosmo sub-licenses its ByFavo™ (remimazolam) US rights to Acacia Pharma, takes an initial 14.1% stake and provides finance for Acacia Pharma’s US expansion [media release]. 10 Jan 2020. https://www.cosmopharma.com/.
PAION AG. About remimazolam. 2020. https://www.paion.com/remimazolam/indikationen/leitsubstanz-remimazolam/. Accessed 2 Mar 2020.
PAION AG. PAION AG: Mundipharma receives market approval for Anerem® (remimazolam) in general anesthesia in Japan [media release]. 23 Jan 2020. https://www.paion.com/.
PAION AG. PAION announces submission of new drug application for remimazolam by its licensee Hana Pharm in South Korea [media release]. 30 Dec 2019. https://www.paion.com/.
Cosmo Pharmaceuticals. FDA accepts filing of NDA for remimazolam [media release]. 10 Jun 2019. https://www.cosmopharma.com.
Acacia Pharma Group plc. Acacia Pharma announces brief extension of FDA review period for NDA for BYFAVO™ [media release]. 12 Mar 2020. https://www.acaciapharma.com/.
PAION AG. PAION announces submission of the marketing authorization application for remimazolam in procedural sedation to the European Medicines Agency [media release]. 20 Nov 2019. https://www.paion.com/.
Wesolowski AM, Zaccagnino MP, Malapero RJ, et al. Remimazolam: pharmacologic considerations and clinical role in anesthesiology. Pharmacotherapy. 2016;36(9):1021–7.
Pambianco DJ, Cash BD. New horizons for sedation: the ultrashort acting benzodiazepine remimazolam. Tech Gastrointest Endosc. 2016;18(1):22–8.
Kilpatrick GJ, McIntyre MS, Cox RF, et al. CNS 7056: a novel ultra-short-acting benzodiazepine. Anesthesiology. 2007;107(1):60–6.
PAION AG. PAION grants Cosmo Pharmaceuticals remimazolam license in the U.S. and Cosmo becomes largest shareholder of PAION AG [media release]. 24 June 2016. https://www.paion.com.
PAION AG. PAION grants remimazolam license for Southeast Asia to Hana Pharm [media release]. 8 Jan 2020. https://www.paion.com.
PAION AG. PAION announces clinical development progress with remimazolam by its partner Hana Pharm in South Korea [media release]. 22 Mar 2018. https://www.paion.com/.
PAION AG. Paion grants exclusive license to Mundipharma for development and commercialization of remimazolam in Japan [media release]. 18 Dec 2017. https://www.paion.com.
PAION AG. PAION grants licence to remimazolam in Canada to Pendopharm [media release]. 9 July 2015. https://www.paion.com/.
PAION AG. PAION AG grants license to R-Pharm for remimazolam [media release]. 30 Oct 2013. https://www.paion.com.
PAION AG. PAION AG grants license for Turkey to add to R-Pharm territory [media release]. 26 Nov 2013. https://www.paion.com/.
TRPharm. PAION AG (ISIN DE000A0B65S3; Frankfurt Stock Exchange Prime Standard: PA8), a specialty pharma company, and R-Pharm, Russia, today announced that they have extended their license agreements for remimazolam to include the "MENA Region" (Middle East andNorth Africa) [media release]. 17 June 2014. https://www.trpharm.com.
PAION AG. PAION AG grants license to Yichang Humanwell for remimazolam in China [media release]. 18 July 2012. https://www.paion.com/.
CeNeS Pharmaceuticals plc. CeNeS licenses new intravenous anaesthetic to Ono in Japan [media release]. 6 Aug 2007. https://www.cenes.com.
PAION AG. PAION's partner Ono decides not to file for regulatory approval of remimazolam in Japan and will return its remimazolam rights for Japan to Paion [media release]. 5 Nov 2014. https://www.paion.de.
PAION AG. PAION AG reports on financial year and financial results 2015 [media release]. 22 Mar 2016. https://www.paion.de.
PAION AG. PAION AG completes acquisition of CeNeS [media release]. 23 June 2008. https://www.paion.de.
CeNeS Pharmaceuticals plc. CeNeS announces preliminary results for the year ended 31 December 2003 [media release]. 20 Apr 2004. https://www.cenes.com.
Ono Pharmaceutical Co L. Ono enters into license agreement with CeNeS Limited [media release]. 6 Aug 2007. https://www.cenes.com.
Google Patents. Dosing regimen for sedation with CNS 7056 (remimazolam). 2020. https://patents.google.com/patent/US10195210B2/en. Accessed 17 Feb 2020.
PAION AG. European Patent Office grants formulation patent for remimazolam in the EU [media release]. 28 Sep 2017. https://www.paion.com.
PAION AG. US Patent & Trademark office grants substance patent for remimazolam besylate [media release]. 12 Feb 2016. https://www.paion.com/.
PAION AG. Japan Patent Office grants dosing patent for remimazolam in Japan [media release]. 13 Oct 2017. https://www.paion.com/.
CeNeS Pharmaceuticals plc. CeNeS is granted patent for short-acting sedatives, including CNS 7056X, by the European Patent Office [media release]. 8 Feb 2006. https://www.cenes.com.
Bevans T, Deering-Rice C, Stockmann C, et al. Inhaled remimazolam potentiates inhaled remifentanil in rodents. Anesth Analg. 2017;124(5):1484–90.
Wiltshire HR, Kilpatrick GJ, Tilbrook GS, et al. A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): part II—population pharmacokinetic and pharmacodynamic modeling and simulation. Anesth Analg. 2012;115(2):284–96.
Antonik LJ, Goldwater DR, Kilpatrick GJ, et al. A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): part I—safety, efficacy, and basic pharmacokinetics. Anesth Analg. 2012;115(2):274–83.
Sheng XY, Liang Y, Yang XY, et al. Safety, pharmacokinetic and pharmacodynamic properties of single ascending dose and continuous infusion of remimazolam besylate in healthy Chinese volunteers. Eur J Clin Pharmacol. 2020;76(3):383–91.
Schuttler J, Eisenried A, Lerch M, et al. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: part I—pharmacokinetics and clinical pharmacodynamics. Anesthesiology. 2020;132(4):636–51.
Eisenried A, Schuttler J, Lerch M, et al. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: part II—pharmacodynamics of electroencephalogram effects. Anesthesiology. 2020;132(4):652–66.
Lohmer LL, Schippers F, Petersen KU, et al. Time-to-event modeling for remimazolam for the indication of induction and maintenance of general anesthesia. J Clin Pharmacol. 2020. https://doi.org/10.1002/jcph.1552.
Sato S, Doi M, Morita K, et al. Remimazolam a new ultra-short acting anesthetic shows similar efficacy and superior hemodynamic stability vs. propofol in general surgery patients with TIVA: results of a randomised, non-inferiority, phase IIb/III trial [abstract no. A5018]. In: 2015 Annual Meeting of the American Society of Anesthesiologists. 2015.
Worthington MT, Antonik LJ, Goldwater DR, et al. A phase Ib, dose-finding study of multiple doses of remimazolam (CNS 7056) in volunteers undergoing colonoscopy. Anesth Analg. 2013;117(5):1093–100.
Kleiman RB, Darpo B, Thorn M, et al. Potential strategy for assessing QT/QTc interval for drugs that produce rapid changes in heart rate: electrocardiographic assessment of the effects of intravenous remimazolam on cardiac repolarization. Br J Clin Pharmacol. 2020.
Freyer N, Knospel F, Damm G, et al. Metabolism of remimazolam in primary human hepatocytes during continuous long-term infusion in a 3-D bioreactor system. Drug Des Dev Ther. 2019;13:1033–47.
Mundipharma Co. Ltd. Remimazolam pharmaceutical interview form. 2020 https://www.info.pmda.go.jp/go/interview/1/770098_11194A1F1029_1_1F.pdf. Accessed 12 Feb 2020.
R-Pharm Group, PAION AG. R-Pharm Group and PAION announces successful completion of remimazolam phase III clinical trial [media release]. 6 Nov 2018. http://www.r-pharm.com/en/.
Hana Pharma. Remizolam, “identical to propofol in general anesthesia, demonstrating reduced side effects” [media release]. 2019. https://www.hanaph.co.kr/.
EU Clinical Trials Register. A randomized, single-blind phase II study evaluating the efficacy, safety and pharmacokinetics of remimazolam in general anesthesia in adult patients undergoing cardiac surgery, including follow-up sedation in the post-anesthesia care unit/intensive care unit. 2016. https://www.clinicaltrialsregister.eu/ctr-search/trial/2013-001113-32/results. Accessed 2 Mar 2020.
Pastis NJ, Yarmus LB, Schippers F, et al. Safety and efficacy of remimazolam compared with placebo and midazolam for moderate sedation during bronchoscopy. Chest. 2019;155(1):137–46.
Hill N, Pastis NJ, Schippers F, et al. Comparing the efficacy and safety of remimazolam versus midazolam based on age and ASA class in sedation for bronchoscopy [abstract no. A1399]. Am J Respir Crit Care Med. 2019;199(9).
Rex DK, Bhandari R, Desta T, et al. A phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2018;88(3):427–37.e6.
ClinicalTrials.gov. Safety and efficacy of remimazolam in ASA III and IV patients undergoing colonoscopy [NCT02532647]. 2015. https://clinicaltrials.gov/. Accessed 12 Feb 2020.
Pambianco DJ, Borkett KM, Riff DS, et al. A phase IIb study comparing the safety and efficacy of remimazolam and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2016;83(5):984–92.
Borkett KM, Riff DS, Schwartz HI, et al. A phase IIa, randomized, double-blind study of remimazolam (CNS 7056) versus midazolam for sedation in upper gastrointestinal endoscopy. Anesth Analg. 2015;120(4):771–80.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Susan Keam is a salaried employee of Adis International Ltd/Springer Nature, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
Enhanced material for this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshare.12017124.
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Keam, S.J. Remimazolam: First Approval. Drugs 80, 625–633 (2020). https://doi.org/10.1007/s40265-020-01299-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-020-01299-8