Abstract
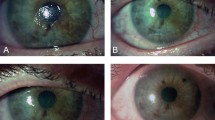
Cenegermin (Oxervate™), an ophthalmic solution containing 20 µg/mL of recombinant human nerve growth factor (rhNGF), is the first drug to be approved for the treatment of neurotrophic keratitis (NK; also referred to as neurotrophic keratopathy). In the registration trials, the majority of adults with moderate or severe NK experienced complete corneal healing after up to 8 weeks of topical cenegermin therapy. The rate of complete corneal healing was generally higher, and that of disease progression was lower, with cenegermin than with vehicle. Although the drug provided no statistically significant benefit over vehicle in terms of corneal sensitivity or visual acuity after 8 weeks of treatment, few patients with corneal healing experienced disease recurrence over 48 weeks of follow-up. Longer-term data are needed to definitively determine the efficacy of cenegermin with respect to these outcomes; further investigation into cenegermin re-treatment following disease recurrence would also be beneficial. Cenegermin had no detrimental impact on health-related quality of life (in contrast to some surgical treatments) and was generally well tolerated. Cenegermin is thus a welcomed non-surgical treatment option approved for this rare and challenging degenerative disease.
Similar content being viewed by others
References
Dua HS, Said DG, Messmer EM, et al. Neurotrophic keratopathy. Prog Retin Eye Res. 2018;66:107–31.
Sacchetti M, Lambiase A. Diagnosis and management of neurotrophic keratitis. Clin Ophthalmol. 2014;8:571–9.
Versura P, Giannaccare G, Pellegrini M, et al. Neurotrophic keratitis: current challenges and future prospects. Eye Brain. 2018;10:37–45.
National Organization for Rare Disorders. Neurotrophic keratitis. 2019. https://rarediseases.org/rare-diseases/neurotrophic-keratitis/. Accessed 2 Mar 2020.
Sheha H, Tighe S, Hashem O, et al. Update on cenegermin eye drops in the treatment of neurotrophic keratitis. Clin Ophthalmol. 2019;13:1973–80.
Aloe L, Rocco ML, Balzamino BO, et al. Nerve growth factor: a focus on neuroscience and therapy. Curr Neuropharmacol. 2015;13(3):294–303.
Lambiase A, Rama P, Bonini S, et al. Topical treatment with nerve growth factor for corneal neurotrophic ulcers. N Engl J Med. 1998;338:1174–80.
Lambiase A, Sacchetti M, Bonini S. Nerve growth factor therapy for corneal disease. Curr Opin Ophthalmol. 2012;23(4):296–302.
Dompé Farmaceutici S.p.A. Oxervate 20 μg/ml eye drops, solution: summary of product characteristics. 2017. https://www.ema.europa.eu/. Accessed 2 Mar 2020.
Bonini S, Lambiase A, Rama P, et al. Phase I trial of recombinant human nerve growth factor for neurotrophic keratitis. Ophthalmol. 2018;125(9):1468–71.
Bonini S, Lambiase A, Rama P, et al. Phase II randomized, double-masked, vehicle-controlled trial of recombinant human nerve growth factor for neurotrophic keratitis. Ophthalmol. 2018;125(9):1332–433.
Ferrari MP, Mantelli F, Sacchetti M, et al. Safety and pharmacokinetics of escalating doses of human recombinant nerve growth factor eye drops in a double-masked, randomized clinical trial. BioDrugs. 2014;28(3):275–83.
Dompé U.S. Inc. Oxervate™ (cenegermin-bkbj) ophthalmic solution for topical ophthalmic use: US prescribing information. 2019. https://www.accessdata.fda.gov/. Accessed 2 Mar 2020.
Pflugfelder SC, Massaro-Giordano M, Perez VL, et al. Topical recombinant human nerve growth factor (cenegermin) for neurotrophic keratopathy: a multicenter randomized vehicle-controlled pivotal trial. Ophthalmol. 2020;127(1):14–6.
European Medicines Agency. Oxervate (cenegermin) public assessment report. 2017. https://www.ema.europa.eu/. Accessed 2 Mar 2020.
Van Nooten F, Harder A, Prisco L, et al. Impact of cenegermin on the quality of life in neurotrophic keratopathy [abstract no. PSS73]. Value Health. 2018;21(Suppl 3):S435.
National Institute for Health and Care Excellence. Cenegermin for treating neurotrophic keratitis. 2018. https://www.nice.org.uk/guidance/ta532. Accessed 2 Mar 2020.
Acknowledgements
During the peer review process, the manufacturer of topical cenegermin was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
Emma Deeks and Yvette Lamb are salaried employees of Adis International Ltd./Springer Nature, are responsible for the article content and declare no relevant conflicts of interest.
Additional information
Enhanced Material
for this Adis Drug Evaluation can be found at https://doi.org/10.6084/m9.figshare.11889078.
The manuscript was reviewed by:A. Di Zazzo, Ophthalmology Complex Operative Unit, Campus Bio-Medico University Hospital, Rome, Italy; K. Jadidi, Vision Health Research Center, Semnan University of Medical Sciences, Semnan, Iran; P. Rama, Vita-Salute San Raffaele University, Milan, Italy; Ophthalmology-Cornea Unit, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Rights and permissions
About this article
Cite this article
Deeks, E.D., Lamb, Y.N. Cenegermin: A Review in Neurotrophic Keratitis. Drugs 80, 489–494 (2020). https://doi.org/10.1007/s40265-020-01289-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-020-01289-w