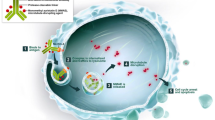
Abstract
The treatment landscape for locally advanced or metastatic urothelial carcinoma has broadened significantly over recent years. New therapeutic options include immunotherapy with checkpoint inhibitors and targeted therapy with erdafitinib. Despite these advances, gaps remain in the selection and sequencing of optimal therapies. Treatment decisions are often influenced by several patient-specific factors such as tolerability and biomarker expression. Following progression while receiving front- and second-line therapies, there is no widely accepted standard of care for patients. Enrollment into a clinical trial is recommended in all lines of therapy for advanced disease. Antibody–drug conjugates have recently emerged as novel therapeutics allowing for targeted delivery of chemotherapeutic agents. Enfortumab vedotin, a nectin-4-targeted antibody conjugated with monomethyl auristatin E, is the first-in-class therapeutic option and has demonstrated unprecedented response rates following progression on chemotherapy and immunotherapy for advanced disease with a tolerable safety profile. As a result, a biologics license application was submitted to the US FDA in July 2019. Ongoing clinical trials are aiming to further establish the role of enfortumab vedotin in urothelial carcinoma. In this article, we highlight the safety and efficacy of enfortumab vedotin for patients with advanced bladder cancer, ongoing clinical trials, clinical pharmacology, and pharmacokinetics.
Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34.
NCCN. The NCCN Clinical Practical Guidelines in Oncology Bladder Cancer (Version 3.2019). 2019; http://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. Accessed 30 July 2019.
National Cancer Institute. SEER stat fact sheets: Bladder cancer. 2019. https://seer.cancer.gov/statfacts/html/urinb.html. Accessed 30 July 2019.
Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507(7492):315–22.
Balversa (erdafitinib) [prescribing information]. Horsham, PA: Janssen Products; April 2019.
Gust KM, McConkey DJ, Awrey S, et al. Fibroblast growth factor receptor 3 is a rational therapeutic target in bladder cancer. Mol Cancer Ther. 2013;12:1245–54.
Hanna KS. A review of immune checkpoint inhibitors for the management of locally advanced or metastatic urothelial carcinoma. Pharmacotherapy. 2017;37(11):1391–405.
Hanna KS. Updates and novel treatments in urothelial carcinoma. J Oncol Pharm Pract. 2019;25(3):648–56.
FDA Grants Breakthrough Therapy Designation to Enfortumab Vedotin for Locally Advanced or Metastatic Urothelial Cancer. 2018. https://www.ascopost.com. Accessed 30 July 2019. https://www.ascopost.com/News/58667.
Diamantis N, Banerji U. Antibody-drug conjugates—an emerging class of cancer treatment. Br J Cancer. 2016;114(4):362–7.
Seattle Genetics and Astellas Announce Submission of Biologics License Application to FDA for Enfortumab Vedotin for Patients with Locally Advanced or Metastatic Urothelial Cancer. 2019. http://investor.seattlegenetics.com/news-releases/news-release-details/seattle-genetics-and-astellas-announce-submission-biologics. Accessed 30 July 2019.
Petrylak DP, Rosenberg JE, Duran I, et al. EV-301: Phase III study to evaluate enfortumab vedotin (EV) versus chemotherapy in patients with previously treated locally advanced or metastatic urothelial cancer (la/mUC). J Clin Oncol. 2019;37(7_suppl): TPS497.
Adcetris (brentuximab vedotin) [prescribing information]. Bothell, WA: Seattle Genetics; November 2018.
Challita-eid PM, Satpayev D, Yang P, et al. Enfortumab vedotin antibody-drug conjugate targeting nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer Res. 2016;76(10):3003–13.
Zhang Y, Zhang J, Shen Q, et al. High expression of nectin-4 is associated with unfavorable prognosis in gastric cancer. Oncol Lett. 2018;15(6):8789–95.
Nishiwada S, Sho M, Yasuda S, et al. Nectin-4 expression contributes to tumor proliferation, angiogenesis and patient prognosis in human pancreatic cancer. J Exp Clin Cancer Res. 2015;34:30.
Rosenberg JE, Heath EI, Veldhuizen PJ, et al. Anti-tumor activity, safety and pharmacokinetics (PK) of ASG-22CE (ASG-22ME; enfortumab vedotin) in a phase I dose escalation trial in patients (Pts) with metastatic urothelial cancer (mUC). J Clin Oncol. 2016;34(15_suppl):4533.
A Study of Escalating Doses of ASG-22CE Given as Monotherapy in Subjects With Metastatic Urothelial Cancer and Other Malignant Solid Tumors That Express Nectin-4—ClinicalTrials.Gov. (n.d.). https://clinicaltrials.gov/ct2/show/NCT02091999. Accessed 30 July 2019.
Rosenberg JE, Sridhar SS, Zhang J, et al. Mature results from EV-101: A phase I study of enfortumab vedotin in patients with metastatic urothelial cancer (mUC). J Clin Oncol. 2019;37(7_supp):377.
Rosenberg JE, Sridhar SS, Zhang J, et al. Updated results from the enfortumab vedotin phase 1 (EV-101) study in patients with metastatic urothelial cancer (mUC). J Clin Oncol. 2018;36(15_suppl):4504.
A study of enfortumab vedotin for patients with locally advanced or metastatic urothelial bladder cancer (EV-201)—ClinicalTrials.Gov. (n.d.). https://clinicaltrials.gov/ct2/show/NCT03219333. Accessed 30 July 2019.
Rosenberg JE, O’donnell PH, Balar AV, et al. Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. J Clin Oncol. 2019;37(29):2592–2600.
A study to evaluate enfortumab vedotin versus (vs) chemotherapy in subjects with previously treated locally advanced or metastatic urothelial cancer (EV-301)—ClinicalTrials.Gov. (n.d.). https://clinicaltrials.gov/ct2/show/NCT03474107. Accessed 30 July 2019.
Bellmunt J, De wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–26.
A study of enfortumab vedotin plus pembrolizumab and/or chemotherapy for patients with urothelial bladder cancer (EV-103)—ClinicalTrials.Gov. (n.d.). https://clinicaltrials.gov/ct2/show/NCT03288545. Accessed 30 July 2019.
Seattle Genetics and Astellas Announce Results from Phase 1 Trial of Investigational Agent Enfortumab Vedotin in Combination with Immune Therapy Pembrolizumab as First-Line Treatment for Advanced Bladder Cancer. 2019. https://investor.seattlegenetics.com/press-releases/news-details/2019/Seattle-Genetics-and-Astellas-Announce-Results-from-Phase-1-Trial-of-Investigational-Agent-Enfortumab-Vedotin-in-Combination-with-Immune-Therapy-Pembrolizumab-as-First-Line-Treatment-for-Advanced-Bladder-Cancer/default.aspx. Accessed 28 Sept 2019.
Takahashi S, Uemura M, Kimura T, et al. A phase I study of enfortumab vedotin in Japanese patients with locally advanced or metastatic urothelial carcinoma. Investig New Drugs. 2019. https://doi.org/10.1007/s10637-019-00844-x. (Epub ahead of print]).
Masters JC, Nickens DJ, Xuan D, et al. Clinical toxicity of antibody drug conjugates: a meta-analysis of payloads. Investig New Drugs. 2018;36:121–35.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to conduct this study or prepare this manuscript.
Conflict of interest
Kirollos Hanna, PharmD, BCPS, BCOP has received consulting fees from Seattle Genetics and is a speaker for Seattle Genetics and Abbvie.
Rights and permissions
About this article
Cite this article
Hanna, K.S. Clinical Overview of Enfortumab Vedotin in the Management of Locally Advanced or Metastatic Urothelial Carcinoma. Drugs 80, 1–7 (2020). https://doi.org/10.1007/s40265-019-01241-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-019-01241-7