Abstract
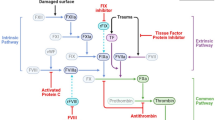
Emicizumab (Hemlibra®), a recombinant, humanized, bispecific monoclonal antibody, restores the function of missing activated factor VIII (FVIII) by bridging FIXa and FX to facilitate effective haemostasis in patients with haemophilia A. Subcutaneous emicizumab is approved in several countries, including in the USA and Japan, for the routine prophylaxis of bleeding episodes in patients with haemophilia A with or without FVIII inhibitors. It is also approved in the EU for the routine prophylaxis of bleeding episodes in patients with haemophilia A with inhibitors or severe haemophilia A without inhibitors. In phase III clinical trials, emicizumab prophylaxis significantly reduced annualized bleeding rates compared with no prophylaxis in adolescents and adults with haemophilia A with or without inhibitors, and prevented or substantially reduced bleeding in children with haemophilia A with or without inhibitors. Emicizumab was also associated with beneficial effects on health-related quality of life and health status, and was generally well tolerated. In view of its convenient route of administration and versatile dosage regimens (maintenance dose of once every 1, 2 or 4 weeks), emicizumab provides an effective and generally well-tolerated alternative to conventional FVIII replacement products for the prophylaxis of bleeding episodes in patients with haemophilia A, regardless of the presence or absence of inhibitors.
Similar content being viewed by others
References
Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1–47.
Pelland-Marcotte MC, Carcao MD. Hemophilia in a changing treatment landscape. Hematol Oncol Clin North Am. 2019;33(3):409–23.
Ljung R, Auerswald G, Benson G, et al. Inhibitors in haemophilia A and B: management of bleeds, inhibitor eradication and strategies for difficult-to-treat patients. Eur J Haematol. 2019;102(2):111–22.
Genentech Inc. Hemlibra® (emicizumab-kxwh) injection, for subcutaneous use: US prescribing information. 2018. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2483adba-fab6-4d1b-96c5-c195577ed071. Accessed 9 Sept 2019.
Chugai Pharmaceutical Co Ltd. Chugai’s Hemlibra® subcutaneous injection receives approval for hemophilia A without inhibitors and extension of dosing interval [media release]. 21 Dec 2018. http://www.chugai-pharm.co.jp/english/news/detail/20181221153002_580.html.
Roche. Hemlibra solution for injection: summary of product characteristics. 2019. http://www.ema.europa.eu/en/documents/product-information/hemlibra-epar-product-information_en.pdf. Accessed 9 Sep 2019.
Kitazawa T, Esaki K, Tachibana T, et al. Factor VIIIa-mimetic cofactor activity of a bispecific antibody to factors IX/IXa and X/Xa, emicizumab, depends on its ability to bridge the antigens. Thromb Haemost. 2017;117(7):1348–57.
Kitazawa T, Igawa T, Sampei Z, et al. A bispecific antibody to factors IXa and X restores factor VIII hemostatic activity in a hemophilia A model. Nat Med. 2012;18(10):1570–4.
Muto A, Yoshihashi K, Takeda M, et al. Anti-factor IXa/X bispecific antibody (ACE910): hemostatic potency against ongoing bleeds in a hemophilia A model and the possibility of routine supplementation. J Thromb Haemost. 2014;12(2):206–13.
Muto A, Yoshihashi K, Takeda M, et al. Anti-factor IXa/X bispecific antibody ACE910 prevents joint bleeds in a long-term primate model of acquired hemophilia A. Blood. 2014;124(20):3165–71.
Sampei Z, Igawa T, Soeda T, et al. Identification and multidimensional optimization of an asymmetric bispecific IgG antibody mimicking the function of factor VIII cofactor activity. PLoS One. 2013;8(2):e57479.
Ogiwara K, Horiuchi H, Nogami K, et al. Assessment of emicizumab-driven clot stability in hemophilia A model [abstract]. Blood. 2018;132(Suppl 1):2478.
Shima M, Hanabusa H, Taki M, et al. Factor VIII-mimetic function of humanized bispecific antibody in hemophilia A. N Engl J Med. 2016;374(21):2044–53.
Shima M, Hanabusa H, Taki M, et al. Long-term safety and efficacy of emicizumab in a phase 1/2 study in patients with hemophilia A with or without inhibitors. Blood Adv. 2017;1(22):1891–9.
Uchida N, Sambe T, Yoneyama K, et al. A first-in-human phase 1 study of ACE910, a novel factor VIII-mimetic bispecific antibody, in healthy subjects. Blood. 2016;127(13):1633–41.
Adamkewicz J, Schmitt C, Calatzis A, et al. Pharmacodynamic data and coagulation biomarkers in persons with hemophilia A (PwHA) with inhibitors: results from the HAVEN 1 emicizumab (ACE910) phase 3 study [abstract no. OC 47.1]. Res Pract Thromb Haemost. 2017;1(Suppl 1):162.
Kiialainen A, Schmitt C, Oldenburg J, et al. Pharmacokinetics and biomarkers in persons with haemophilia a (PwHA) without FVIII inhibitors receiving emicizumab once weekly in the phase 3 HAVEN 3 study [abstract no. P022]. Haemophilia. 2019;25(Suppl 1):46–7.
Kiialainen A, Schmitt C, Adamkewicz JI, et al. Pharmacokinetics and biomarkers in persons with haemophilia a (PwHA) receiving emicizumab every 2 or 4 weeks [abstract no. P021]. Haemophilia. 2019;25(Suppl 1):45–6.
Oldenburg J, Mahlangu JN, Kim B, et al. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med. 2017;377(9):809–18.
Mahlangu J, Oldenburg J, Paz-Priel I, et al. Emicizumab prophylaxis in patients who have hemophilia A without inhibitors. N Engl J Med. 2018;379(9):811–22.
Pipe S, Shima M, Lehle M, et al. Efficacy, safety, and pharmacokinetics of emicizumab prophylaxis given every 4 weeks in people with haemophilia A (HAVEN 4): a multicentre, open-label, non-randomised phase 3 study. Lancet Haematol. 2019;6(6):e295–305.
Jimenez-Yuste V, Klamroth R, Castaman G, et al. A single-arm, multicentre, open-label, phase III clinical trial to evaluate the safety and tolerability of prophylactic emicizumab in persons with haemophilia A (PwHA) with FVIII inhibitors (STASEY): interim analysis results [abstract no. OC 60.3]. Res Pract Thromb Haemost. 2019;3(Suppl 1):116–7.
Young G, Liesner R, Sidonio RF Jr, et al. Emicizumab prophylaxis provides flexible and effective bleed control in children with hemophilia A with inhibitors: results from the HAVEN 2 study [abstract]. Blood. 2018;132(Suppl 1):632.
Shima M, Nogami K, Nagami S, et al. Every 2 weeks or every 4 weeks subcutaneous injection of emicizumab in pediatric patients with severe hemophilia a without inhibitors: a multi-center, open-label study in Japan (HOHOEMI study) [abstract]. Blood. 2018;132(Suppl 1):1186.
Callaghan MU, Kuebler PJ, Gao L, et al. Characterization of the impact of prior ITI on patient outcomes in HAVEN1 [abstract]. Am J Hematol. 2018;93(9):e10–1.
Mancuso ME, Oldenburg J, Callaghan M, et al. Emicizumab prophylaxis in adolescent/adult patients with haemophilia A previously receiving episodic or prophylactic bypassing agent treatment: updated analyses from the HAVEN 1 study [abstract no. BSH18-PO-146]. Br J Haematol. 2018;181(Suppl 1):127.
Oldenburg J, Mahlangu JN, Bujan W, et al. The effect of emicizumab prophylaxis on health-related outcomes in persons with haemophilia A with inhibitors: HAVEN 1 study. Haemophilia. 2019;25(1):33–44.
Skinner M, Negrier C, Paz-Priel I, et al. Emicizumab prophylaxis improves long-term physical health scores in persons with haemophilia A (PwHA) with and without inhibitors: update from the HAVEN 3 and HAVEN 4 studies [abstract no. PB0698]. Res Pract Thromb Haemost. 2019;3(Suppl 3):328–9.
Jimenez-Yuste V, Shima M, Paz-Priel I, et al. Preference for emicizumab over prior factor treatments: results from the HAVEN 3 and HAVEN 4 studies [abstract]. Blood. 2018;132(Suppl 1):1187.
Mancuso ME, Mahlangu J, Sidonio RF, et al. Emicizumab prophylaxis in paediatric persons with haemophilia A (PWHA) with inhibitors: impact on health-related outcomes and caregiver burden in the HAVEN 2 study [abstract no. OR10]. Haemophilia. 2018;24(Suppl 1):28–9.
Santagostino E, Oldenburg J, Chang T, et al. Surgical experience from four phase III studies (HAVEN 1-4) of emicizumab in persons with haemophilia A (PwHA) with or without FVIII inhibitors [abstract no. OC 60.1]. Res Pract Thromb Haemost. 2019;3(Suppl 1):115.
Callaghan M, Negrier C, Paz-Priel I, et al. Emicizumab treatment is efficacious and well tolerated long term in persons with haemophilia A (PwHA) with or without FVIII inhibitors: pooled data from four HAVEN studies [abstract no. OC 60.2]. Res Pract Thromb Haemost. 2019;3(Suppl 1):116.
Levy GG, Asikanius E, Kuebler P, et al. Safety analysis of rFVIIa with emicizumab dosing in congenital hemophilia A with inhibitors: experience from the HAVEN clinical program. J Thromb Haemost. 2019. https://doi.org/10.1111/jth.14491.
Paz-Priel I, Chang T, Asikanius E, et al. Immunogenicity of emicizumab in people with hemophilia A (PwHA): results from the HAVEN 1-4 studies [abstract]. Blood. 2018;132(Suppl 1):633.
Rocino A, Franchini M, Coppola A. Treatment and prevention of bleeds in haemophilia patients with inhibitors to factor VIII/IX. J Clin Med. 2017;6(4):46.
National Hemophilia Foundation. Recommendation on the use and management of emicizumab-kxwh (Hemlibra®) for hemophilia A with and without inhibitors. 2018. http://www.hemophilia.org. Accessed 9 Sept 2019.
Collins PW, Liesner R, Makris M, et al. Treatment of bleeding episodes in haemophilia A complicated by a factor VIII inhibitor in patients receiving emicizumab. Interim guidance from UKHCDO inhibitor working party and executive committee. Haemophilia. 2018;24(3):344–7.
Scott LJ, Kim ES. Emicizumab-kxwh: first global approval. Drugs. 2018;78(2):269–74.
Cassis FR, Querol F, Forsyth A, et al. Psychosocial aspects of haemophilia: a systematic review of methodologies and findings. Haemophilia. 2012;18(3):e101–14.
Coppola A, Cerbone AM, Mancuso G, et al. Confronting the psychological burden of haemophilia. Haemophilia. 2011;17(1):21–7.
Nogami K, Soeda T, Matsumoto T, et al. Routine measurements of factor VIII activity and inhibitor titer in the presence of emicizumab utilizing anti-idiotype monoclonal antibodies. J Thromb Haemost. 2018;16(7):1383–90.
Nogami K, Matsumoto T, Tabuchi Y, et al. Modified clot waveform analysis to measure plasma coagulation potential in the presence of the anti-factor IXa/factor X bispecific antibody emicizumab. J Thromb Haemost. 2018;16(6):1078–88.
Adamkewicz J, Kim B, Steinbuesch D, et al. Measurement of FVIII inhibitor titer using a chromogenic Bethesda assay (CBA) in the presence of emicizumab (ACE910), a humanized bispecific antibody mimicking FVIIIa cofactor function [abstract]. Haemophilia. 2017;23(Suppl 3):3–4.
Adamkewicz J, Chen DC, Paz-Priel I. Effects and interferences of emicizumab, a humanised bispecific antibody mimicking activated factor VIII cofactor function, on coagulation assays. Thromb Haemost. 2019;119:1084–93.
US National Institutes of Health (2019) http://www.clinicaltrials.gov. Accessed 9 Sept 2019
Cafuir L, Kruse-Jarres R, Mancuso ME, et al. Emicizumab for hemophilia A without inhibitors. Expert Rev Hematol. 2019;12(7):515–24.
Escuriola-Ettingshausen C, Sidonio RF Jr, Oldenburg J, et al. Modern treatment of inhibitor-positive patients with haemophilia A (MOTIVATE)—an international observational study [abstract no. PB1406]. Res Pract Thromb Haemost. 2019;3(Suppl 1):423.
Chen SL. Economic costs of hemophilia and the impact of prophylactic treatment on patient management. Am J Manag Care. 2016;22(Suppl 5):s126–33.
Institute for Clinical and Economic Review. Emicizumab for hemophilia A with inhibitors: effectiveness and value. 2018. http://icer-review.org/wp-content/uploads/2017/08/ICER_Hemophilia_Final_Evidence_Report_041618.pdf. Accessed 9 Sept 2019.
Mahajerin A, Zhou ZY, Raimundo K, et al. Model of short and long-term outcomes of emicizumab prophylaxis treatment for persons with hemophilia A [abstract]. Blood. 2018;132(Suppl 1):3511.
Sidonio RF, Patel A, Corman S, et al. Model of the impact of delayed inhibitor development on cumulative breakthrough bleeds and costs in persons with hemophilia a receiving emicizumab prophylaxis [abstract]. Blood. 2018;132(Suppl 1):4710.
Acknowledgements
During the review process, the manufacturer of emicizumab was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Hannah Blair is a salaried employee of Adis International Ltd/Springer Nature, is responsible for the article content and declares no relevant conflicts of interest.
Funding
The preparation of this review was not supported by any external funding.
Additional information
Enhanced material for this Adis Drug Evaluation can be found at https://doi.org/10.6084/m9.figshare.9784490.
The manuscript was reviewed by:J. N. Mahlangu, Haemophilia Comprehensive Care Centre, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences, University of the Witwatersrand and NHLS, Johannesburg, South Africa; M. E. Mancuso, Fondazione IRCCS Ca’ Granda, Ospedale Maggiore Policlinico Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Milan, Italy; J. Oldenburg, University Clinic Bonn, Institute for Experimental Haematology and Transfusion Medicine, Bonn, Germany.
Rights and permissions
About this article
Cite this article
Blair, H.A. Emicizumab: A Review in Haemophilia A. Drugs 79, 1697–1707 (2019). https://doi.org/10.1007/s40265-019-01200-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-019-01200-2