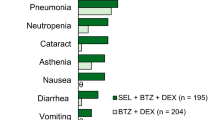
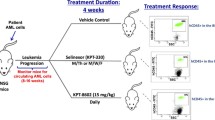
Abstract
Selinexor (XPOVIO™) is a first-in-class, oral, small molecule Exportin-1 (XPO1) inhibitor that is being developed by Karyopharm Therapeutics for the treatment of cancer. Selinexor (in combination with dexamethasone) received accelerated approval in the USA in July 2019 for the treatment of adult patients with relapsed or refractory multiple myeloma (RRMM). Selinexor is also undergoing clinical development in a wide range of haematological and solid cancers. This article summarizes the milestones in the development of selinexor leading to this first approval for RRMM.
Similar content being viewed by others
References
Karyopharm Therapeutics Inc. XPOVIO™ (selinexor) tablets, for oral use: US prescribing information; 2019. https://www.fda.gov. Accessed 2 Aug 2019.
Gounder MM, Zer A, Tap WD, et al. Phase IB study of selinexor, a first-in-class inhibitor of nuclear export, in patients with advanced refractory bone or soft tissue sarcoma. J Clin Oncol. 2016;34(26):3166–74.
Abdul Razak AR, Mau-Soerensen M, Gabrail NY, et al. First-in-class, first-in-human phase I study of selinexor, a selective inhibitor of nuclear export, in patients with advanced solid tumors. J Clin Oncol. 2016;34(34):4142–50.
Gandhi UH, Senapedis W, Baloglu E, et al. Clinical implications of targeting XPO1-mediated muclear export in multiple myeloma. Clin Lymphoma Myeloma Leuk. 2018;18(5):335–45.
Karyopharm Therapeutics. Karyopharm announces FDA approval of XPOVIO™ (selinexor) for the treatment of patients with relapsed or refractory multiple myeloma [media release]. 3 Jul 2019. http://www.karyopharm.com.
Karyopharm Therapeutics. Karyopharm’s selinexor receives fast track designation from FDA for the treatment of patients with penta-refractory multiple myeloma [media release]. 10 Apr 2018. http://www.karyopharm.com.
Karyopharm Therapeutics. Karyopharm and Antengene sign exclusive license agreement to develop and commercialize selinexor, eltanexor, verdinexor and KPT-9274 in China and other regions in Asia [media release]. 24 May 2018. http://www.karyopharm.coms.
Karyopharm Therapeutics. Karyopharm and Ono Pharmaceutical Co. Ltd. sign exclusive license agreement to develop and commercialize selinexor and KPT-8602 in Japan and other countries in Asia [media release]. 12 Oct 2017. http://www.karyopharm.com.
Karyopharm Therapeutics. Ivy Brain Tumor Center and Karyopharm Therapeutics collaborate to develop tissue-based clinical trial for brain cancer [media release]. 11 Jul 2019. http://www.karyopharm.com.
Karyopharm Therapeutics. Karyopharm Therapeutics Inc. and Katholieke Universiteit Leuven sign exclusive collaboration for the discovery of novel CRM1/Exportin 1 inhibitors [media release]. 11 Oct 2011. http://www.karyopharm.com.
Veristat. Veristat and Karyopharm Therapeutics Inc. enter into preferred provider agreement for oncology and novel therapeutic advancement [media release]. 15 Jun 2014. http://www.karyopharm.com.
Alexander TB, Lacayo NJ, Choi JK, et al. Phase I study of selinexor, a selective inhibitor of nuclear export, in combination with fludarabine and cytarabine, in pediatric relapsed or refractory acute leukemia. J Clin Oncol. 2016;34(34):4094–101.
Conforti F, Zhang X, Rao G, et al. Therapeutic effects of XPO1 inhibition in thymic epithelial tumors. Cancer Res. 2017;77(20):5614–27.
Taylor-Kashton C, Lichtensztejn D, Baloglu E, et al. XPO1 inhibition preferentially disrupts the 3D nuclear organization of telomeres in tumor cells. J Cell Physiol. 2016;231(12):2711–9.
Taylor J, Coleman M, Alvarez K, et al. Selinexor, a first-in-class XPO1 inhibitor, is efficacious and tolerable in patients with myelodysplastic syndromes refractory to hypomethylating agents [abstract]. Blood. 2018;132(Suppl. 1):233.
Argueta C, Kashyap T, Klebanov B, et al. Selinexor synergizes with dexamethasone to repress mTORC1 signaling and induce multiple myeloma cell death. Oncotarget. 2018;9(39):25529–44.
Turner JG. The synergistic effect of melphalan and XPO1 inhibition in preclinical models of multiple myeloma [abstract no. 4049]. Cancer Res. 2017;77(13 Suppl.).
Rosebeck S, Alonge MM, Kandarpa M, et al. Synergistic myeloma cell death via novel intracellular activation of caspase-10-dependent apoptosis by carfilzomib and selinexor. Mol Cancer Ther. 2016;15(1):60–71.
Hing ZA, Mantel R, Beckwith KA, et al. Selinexor is effective in acquired resistance to ibrutinib and synergizes with ibrutinib in chronic lymphocytic leukemia. Blood. 2015;125(20):3128–32.
Todaro M, Griggio V, Vitale C, et al. Selinexor (KPT-330) in combination with chemotherapy or idelalisib elicits a synergistic cytotoxic effect in primary CLL cells, also overcoming intrinsic and stromal cells-mediated fludarabine resistance [abstract no. P0087]. Haematologica. 2018;103(Suppl. 3):S110.
Muqbil I, Aboukameel A, Elloul S, et al. Anti-tumor activity of selective inhibitor of nuclear export (SINE) compounds, is enhanced in non-Hodgkin lymphoma through combination with mTOR inhibitor and dexamethasone. Cancer Lett. 2016;383(2):309–17.
Fischer MA, Friedlander S, Hogdal L, et al. Combination of selective inhibitor of nuclear export (SINE) compounds, selinexor and KPT-8602, with venetoclax (ABT-199) displays enhanced activity in leukemia and large cell lymphoma [abstract]. Blood. 2016;128(22):3949.
DeSisto J, Flannery P, Kashyap T, et al. Synergistic effects of the XPO1 inhibitor selinexor with proteasome inhibitors in pediatric high-grade glioma and diffuse intrinsic pontine glioma [abstract no. 1946]. Cancer Res. 2017;77(13 Suppl.).
Kashyap T, Muqbil I, Aboukameel A, et al. Combination of selinexor and the proteasome inhibitor, bortezomib shows synergistic cytotoxicity in diffuse large B-cells lymphoma cells in vitro and in vivo [abstract]. Blood. 2016;128(22):4131.
Arango NP, Evans K, Zhao M, et al. Nuclear export inhibitor selinexor (KPT-330) demonstrates anti-tumor efficacy alone and in combination with chemotherapy in multiple breast cancer models [abstract no. 3075]. Cancer Res. 2016;76(14 Suppl.).
Kashyap T, Argueta C, Unger T, et al. Selinexor reduces the expression of DNA damage repair proteins and sensitizes cancer cells to DNA damaging agents. Oncotarget. 2018;9(56):30773–86.
Turner JG, Kashyap T, Dawson JL, et al. XPO1 inhibitor combination therapy with bortezomib or carfilzomib induces nuclear localization of IkBa and overcomes acquired proteasome inhibitor resistance in human multiple myeloma. Oncotarget. 2016;7(48):78896–909.
Turner JG, Dawson JL, Grant S, et al. Treatment of acquired drug resistance in multiple myeloma by combination therapy with XPO1 and topoisomerase II inhibitors. J Hematol Oncol. 2016;9(1):73.
Muz B, Azab F, de la Puente P, et al. Selinexor overcomes hypoxia-induced drug resistance in multiple myeloma. Transl Oncol. 2017;10(4):632–40.
Zhong Y, El-Gamal D, Dubovsky JA, et al. Selinexor suppresses downstream effectors of B-cell activation, proliferation and migration in chronic lymphocytic leukemia cells. Leukemia. 2014;28(5):1158–63.
Kashyap T, Argueta C, Aboukameel A, et al. Selinexor, a selective inhibitor of nuclear export (SINE) compound, acts through NF-kappaB deactivation and combines with proteasome inhibitors to synergistically induce tumor cell death. Oncotarget. 2016;7(48):78883–95.
Lagana A, Park S, Edwards D, et al. E2F1 is a biomarker of selinexor resistance in relapsed/refractory multiple myeloma patients [abstract]. Blood. 2018;132(Suppl. 1):3216.
Crochiere M, Kashyap T, Kalid O, et al. Deciphering mechanisms of drug sensitivity and resistance to selective inhibitor of nuclear export (SINE) compounds. BMC Cancer. 2015;15:910.
Broijl A, Asselbergs E, Minnema M, et al. A phase II study of selinexor (KPT-330) combined with bortezomib and dexamethasone (SVD) for induction and consolidation for patients with progressive or refractory multiple myeloma: the Selvedex trial [abstract no. PS1338 + poster ]. HemaSphere. 2018;2(Suppl. 2):611–2.
Chen C, Siegel D, Gutierrez M, et al. Safety and efficacy of selinexor in relapsed or refractory multiple myeloma and Waldenstrom macroglobulinemia. Blood. 2018;131(8):855–63.
Vogl DT, Dingli D, Cornell RF, et al. Selective inhibition of nuclear export with oral selinexor for treatment of relapsed or refractory multiple myeloma. J Clin Oncol. 2018;36(9):859–66.
Chari A, Vogl DT, Dimopoulos MA, et al. Results of the pivotal STORM study (part 2) in penta-refractory multiple myeloma (MM): deep and durable responses with oral selinexor plus low dose dexamethasone in patients with penta exposed and triple class-refractory MM [abstract]. Blood. 2018;132(Suppl. 1):598.
Jagannath S, Vogl DT, Dimopoulos MA, et al. Phase 2b results of the STORM study: oral selinexor plus low dose dexamethasone (Sd) in patients with penta-refractory myeloma (penta-MM) [abstract no. MM-255]. Clin Lymphoma Myeloma Leuk. 2018;18(Suppl. 1):S249–50.
Richardson P, Jagannath S, Chari A, et al. Overall survival (OS) with oral selinexor plus low dose dexamethasone (Sd) in patients with triple class refractory-multiple myeloma (TCR-MM) [abstract]. J Clin Oncol. 2019;37(15 Suppl.):8014.
White DJ, Bahlis NJ, Venner CP, et al. A phase IB/II trial of selinexor combined with lenalidomide and low dose dexamethasone in patients with relapsed/refractory multiple myeloma [abstract]. Blood. 2017;130(Suppl. 1):1861.
Bahlis NJ, Sutherland H, White D, et al. Selinexor plus low-dose bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma. Blood. 2018;132(24):2546–54.
Gasparetto C, Lentzsch S, Schiller G, et al. Safety and efficacy of the combination of selinexor, daratumumab, and dexamethasone (SDd) in patients with multiple myeloma (MM) previously exposed to proteasome inhibitors and immunomodulatory drugs. HemaSphere. 2019;3:740.
Chen C, Gasparetto C, White D, et al. Selinexor plus pomalidomide and low dose dexamethasone (SPd) in patients with relapsed or refractory multiple myeloma [abstract no. 3199 + oral]. In: 24th Congress of the European Hematology Association; 2019.
Gasparetto C, Lentzsch S, Schiller G, et al. A phase 1b/2 study of selinexor, carfilzomib, and dexamethasone (SKd) in relapsed/refractory multiple myeloma (RRMM) [abstract no. 3423 + oral]. In: 24th Congress of the European Hematology Association; 2019.
Jakubowiak AJ, Jasielec JK, Rosenbaum CA, et al. Phase 1 study of selinexor plus carfilzomib and dexamethasone for the treatment of relapsed/refractory multiple myeloma. Br J Haematol. 2019. https://doi.org/10.1111/bjh.15969.
Gasparetto C, Lentzsch S, Schiller G, et al. A phase 1b/2 study of selinexor, carfilzomib, and dexamethasone (skd) in relapsed/refractory multiple myeloma (RRMM): PS1414 [abstract no. PS1414]. HemaSphere. 2019;3(Suppl. 1):650.
Baz R, Zonder JA, Shain KH, et al. Phase I/II study of liposomal doxorubicin (DOX) in combination with selinexor (SEL) and dexamethasone (DEX) for relapsed and refractory multiple myeloma (RRMM) [abstract]. Blood. 2017;130(Suppl. 1):3095.
Kuruvilla J, Savona M, Baz R, et al. Selective inhibition of nuclear export with selinexor in patients with non-Hodgkin lymphoma. Blood. 2017;129(24):3175–83.
Kalakonda N, Cavallo F, Follows G, et al. A phase 2b study of selinexor in patients with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) [abstract no. 031]. Hematol Oncol. 2019;37(S2):62–4.
Garzon R, Savona M, Baz R, et al. A phase 1 clinical trial of single-agent selinexor in acute myeloid leukemia. Blood. 2017;129(24):3165–74.
Place AE, Blonquist TM, Stieglitz E, et al. Phase I study of the selinexor in relapsed/refractory childhood acute leukemia [abstract]. Blood. 2018;132(Suppl. 1):1405.
Daver NG, Assi R, Kantarjian HM, et al. Final results of phase I/II study of selinexor (SEL) with sorafenib in patients (pts) with relapsed and/or refractory (R/R) FLT3 mutated acute myeloid leukemia (AML) [abstract no. 1441]. Blood. 2018;132(Suppl 1).
Fiedler W, Heuser M, Chromik J, et al. Phase II results of ara-C and idarubicin in combination with the selective inhibitor of nuclear export (SINE) compound selinexor (KPT-330) in patients with relapsed or refractory AML [abstract]. Blood. 2016;128(22):341.
Wang AY, Weiner H, Green M, et al. A phase I study of selinexor in combination with high-dose cytarabine and mitoxantrone for remission induction in patients with acute myeloid leukemia. J Hematol Oncol. 2018;11(4):1–10.
Bhatnagar B, Klisovic RB, Walker AR, et al. A phase 1 clinical trial of selinexor in combination with decitabine in patients with newly diagnosed and relapsed or refractory acute myeloid leukemia [abstract]. Blood. 2016;128(22):1651.
Bhatnagar B, Walker AR, Mims AS, et al. Phase 1 study of selinexor plus mitoxantrone, etoposide, and cytarabine in acute myeloid leukemia [abstract]. J Clin Oncol. 2018;36(15 Suppl.).
Uy GL, Rettig MP, Fletcher T, et al. Selinexor in combination with cladribine, cytarabine and G-CSF for relapsed or refractory AML [abstract]. Blood. 2017;130(Suppl. 1):816.
Gounder MM, Somaiah N, Attia S, et al. Phase 2 results of selinexor in advanced dedifferentiated (DDLS) liposarcoma (SEAL) study: A phase 2/3, randomized, double blind, placebo controlled cross-over study [abstract no. 11512]. J Clin Oncol. 2018;36(15 Suppl. 1).
Malone ER, Al-Ezzi E, Gupta AA, et al. Phase 1b study of selinexor, a first in class selective inhibitor of nuclear export (SINE) compound, in combination with doxorubicin in patients (pts) with locally advanced or metastatic soft tissue sarcoma (STS) [abstract no. 11562]. J Clin Oncol. 2018;36(15 Suppl.).
Anonymous. Results of a phase 2 trial of selinexor, an oral selective inhibitor of nuclear export (SINE) in 114 patients with gynaecological cancers. Clin Adv Hematol Oncol. 2016;14(12 Suppl.14):8-10.
Makker V, Boucicaut N, Cadoo KA, et al. A phase 1 study of selinexor (S) in combination with paclitaxel (P) and carboplatin (C) in patients (pts) with advanced ovarian (OC) or endometrial cancers (EC) [abstract no. 970P]. Ann Oncol. 2017;28(Suppl. 5):v346.
Lassman AB, Wen PY, Van Den Bent MJ, et al. Efficacy and safety of selinexor in recurrent glioblastoma [abstract no. 2005]. J Clin Oncol. 2019;37(15 Suppl.).
Kendra KL, Watson R, Lesinski GB. Selinexor, a selective inhibitor of nuclear export (SINE), in patients with unresectable melanoma [abstract no. e21014]. J Clin Oncol. 2017;35(15 Suppl.).
Shafique M, Ismail-Khan R, Extermann M, et al. A Phase II trial of selinexor (KPT-330) for metastatic triple-negative breast cancer. Oncologist. 2019. https://doi.org/10.1634/theoncologist.2019-0231.
Nilsson S, Stein A, Rolfo C, et al. Selinexor (KPT-330), an oral selective inhibitor of nuclear export (SINE) compound, in combination with FOLFOX in patients with metastatic colorectal cancer (mCRC)-final results of the phase I SENTINEL trial [abstract no. V368]. Oncol Res Treat. 2018;41(Suppl. 4):122.
Wei XX, Siegel AP, Aggarwal R, et al. A Phase II trial of selinexor, an oral selective inhibitor of nuclear export compound, in abiraterone- and/or enzalutamide-refractory metastatic castration-resistant prostate cancer. Oncologist. 2018;23(6):656–e64.
Delimpasi S, Pour L, Auner HW, et al. A phase 3 randomized, controlled, open-label study of selinexor, bortezomib, and dexamethasone (SVd) versus bortezomib and dexamethasone (Vd) in patients with relapsed or refractory multiple myeloma (RRMM) [abstract no. TPS8056]. J Clin Oncol. 2018;36(15 Suppl.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the author on the basis of scientific completeness and accuracy. Yahiya Y. Syed is a salaried employee of Adis International Ltd/Springer Nature, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
Additional information for this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshare.9586013.
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Syed, Y.Y. Selinexor: First Global Approval. Drugs 79, 1485–1494 (2019). https://doi.org/10.1007/s40265-019-01188-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-019-01188-9