Abstract
Allogeneic hematopoietic stem cell transplantation (Allo-HSCT) is a curative treatment for many hematological malignant and non-malignant diseases. A major complication of the procedure is the donor T-cell-mediated graft-versus-host disease (GvHD). GvHD accounts for about 10% of early mortality after transplantation. GVHD is also the major cause of morbidity and disability in the late follow-up phase of transplanted patients, mainly because of the low response to first-line steroids, and the lack of efficient second-line standard treatments. The increasing knowledge regarding GVHD pathogenesis provides new pharmacological targets, potentially exploitable in clinical practice, in order to prevent and treat this complication. This review provides a description of GVHD pathogenesis, with a focus on the central role of the Janus kinase-related mechanisms. The first inflammatory innate-immunity response is triggered by a JAK/STAT dependent pathway, and JAK inhibition impairs antigen-presenting cell differentiation and activation and downregulates the expression of signals for T-cell triggering. The chronic evolution of alloreactivity, characterized by the long-term maintenance of inflammation and fibrosis, is also dependent on JAK/STAT activation. Based on preclinical data, we reviewed the rationale behind the clinical use of JAK-inhibitors in GVHD, presenting available results of clinical trials and reports, and looked at future implementation of this new promising treatment approach.

Similar content being viewed by others
References
D'Souza A, Fretham C. Current Uses and Outcomes of Hematopoietic Cell Transplantation (HCT): CIBMTR Summary Slides. 2018. Available at https://www.cibmtr.org.
Luznik L, Bolanos-Meade J, Brodsky R, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115(16):3224–30.
Socie G, Schmoor C, Zander AR, et al. Chronic graft-versus-host disease: long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti-T-cell globulin ATG-Fresenius. Blood. 2011;117(23):6375–82.
Kroger N, Solano C, Pini M, et al. Antilymphocyte globulin for prevention of chronic graft-versus-host disease. N Engl J Med. 2016;374(1):43–53.
Flowers ME, Inamoto Y, Carpenter PA, et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood. 2011;117(11):3214–9.
Cooke KR, Luznik L, Sarantopoulos S, et al. The biology of chronic graft-versus-host disease: a task force report from the national institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2016;23(2):211–34.
Arai S, Arora M, Pavletic SZ, et al. Increasing incidence of chronic graft-versus-host disease in allogeneic transplantation: a report from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2015;21(2):266–74.
Martin PJ, Rizzo JD, Wingard JR, et al. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2012;18:1150–63.
Dignan L, Amrolia P, Clark A, et al. Diagnosis and management of chronic graft-versus-host disease. Br J Haematol. 2012;158:46–61.
MacMillan ML, Weisdorf DJ, Wagner JE, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8:387–94.
Martin PJ, Schoch G, Fisher L, et al. A retrospective analysis of therapy for acute graft-versus-host disease: secondary treatment. Blood. 1991;77:1821–8.
Shapira MY, Klimov A, Bloom AI, et al. Regional intra-arterial steroid treatment in 120 patients with steroid-resistant or -dependent GvHD. Bone Marrow Transplant. 2017;52:1416–22.
Ruutu T, Gratwohl A, de Witte T, et al. Prophylaxis and treatment of GVHD: EBMT-ELN working group recommendations for a standardized practice. Bone Marrow Transplant. 2014;49:168–73.
Benito AI, Furlong T, Deeg HJ, et al. Sirolimus (rapamycin) for the treatment of steroid-refractory acute graft-versus-host disease. Transplantation. 2001;72(12):1924–9.
Perfetti P, Carlier P, Bacigalupo A, et al. Extracorporeal photopheresis for the treatment of steroid refractory acute GVHD. Bone Marrow Transplant. 2008;42(9):609–17.
Furlong T, Martin P, Nash RA, et al. Therapy with mycophenolate mofetil for refractory acute and chronic GVHD. Bone Marrow Transplant. 2009;44(11):739–48.
Arai S, Margolis J, Vogelsang GB, et al. Poor outcome in steroid-refractory graft-versus-host disease with antithymocyte globulin treatment. Biol Blood Marrow Transplant. 2002;8(3):155–60.
Miklos D, Cutler CS, Jaglowski S, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood. 2017;130(21):2243–50.
Ferrara JLM, Levine JE, Holler E, et al. Graft-versus-host disease. Lancet. 2009;373(9674):1550–61.
Xun CQ, Thompson JS, Widmer MB, et al. Effect of total body irradiation, busulfan-cyclophosphamide, or cyclophosphamide conditioning on inflammatory cytokine release and development of acute and chronic graft-versus-host disease in H-2-incompatible transplanted SCID mice. Blood. 1994;83:2360–7.
Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000;95:2754–9.
Schwab L, Goroncy L, Zeiser R, et al. Neutrophil granulocytes recruited upon translocation of intestinal bacteria enhance graft-versus-host disease via tissue damage. Nat Med. 2014;20(6):648–54.
Choi SW, Kitko CL, Braun T, et al. Change plasma tumor necrosis factor recept-1 levels in the first week post-myeloablative allogeneic transplant correlated with severity and incidence of GVHD and survival. Blood. 2008;112(4):1539–42.
Shlomchik WD, Couzens MS, Emerson SG, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–5.
Duffner UA, Maeda Y, Teshima T. Host dendritic cells alone are sufficient to initiate acute graft versus-host disease. J Immunol. 2004;172:7393–8.
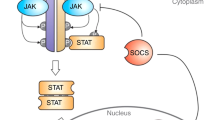
Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228(1):273–87.
Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140(6):845–58.
Hoffmann P, Ermann J, Strober S. Donor-type CD4+ CD25+ regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389–99.
Yang YG, Wang H, Dey BR, et al. Role of Interferon-gamma in GVHD and GVL. Cell Mol Immunol. 2005;2(5):323–9.
Serody JS, Hill GR. The IL-17 differentiation pathway and its role in transplant outcome. Biol Blood Marrow Transplant. 2012;18(1 suppl):S56–61.
Wysocki CA, Panoskaltsis-Mortari A, Serody JS, et al. Leukocyte migration and graft-versus-host disease. Blood. 2005;105:4191–9.
Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol. 2007;25:139–70.
Brown GR, Lee E, Thiele DL. TNF-TNFR2 interactions are critical for the development of intestinal graft-versus-host disease in MHC class II-disparate (C57BL/6J → C57BL/6Jx bm12) F1 mice. J Immunol. 2002;168:3065–71.
Schroeder MA, Choi J, Dipersio JF, et al. The Role of Janus kinase signaling in graft-versus-host disease and graft versus leukemia. Biol Blood Marrow Transplant. 2018;24(6):1125–34.
Zhang Y, Hexner E, Emerson SG, et al. CD4+ T cells generated de novo from donor hemopoietic stem cells mediate the evolution from acute to chronic graft-versus-host disease. J Immunol. 2007;179(5):3305–14.
Srinivasan M, Flynn R, Blazar BR, et al. Donor B-cell alloantibody deposition and germinal center formation are required for the development of murine chronic GVHD and bronchiolitis obliterans. Blood. 2012;119(6):1570–80.
Sarantopoulos S, Stevenson KE, Kim HT, et al. High levels of B-cell activating factor in patients with active chronic graft-versus-host disease. Clin Cancer Res. 2007;13(20):6107–14.
Cooke KR, Luznik L, Sarantopoulos S, et al. The biology of chronic graft-versus-host disease: a task force report from the National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2017;23:211–34.
Ghoreschi K, Laurence A, O’Shea JJ, et al. Janus kinases in immune cell signaling. Immunol Rev. 2009;228(1):273–87.
Al-Shami A, Naccache PH. Granulocyte-macrophage colony-stimulating factor-activated signaling pathways in human neutrophils. Involvement of Jak2 in the stimulation of phosphatidylinositol 3-kinase. J Biol Chem. 1999;274(9):5333–8.
Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–52.
Heine A, Held SA, Brossart P, et al. The JAK-inhibitor ruxolitinib impairs dendritic cell function in vitro and in vivo. Blood. 2013;122(7):1192–202.
Kubo S, Yamaoka K, Tanaka Y, et al. The JAK inhibitor, tofacitinib, reduces the T cell stimulatory capacity of human monocyte-derived dendritic cells. Ann Rheum Dis. 2014;73(12):2192–8.
Choi J, Cooper ML, DiPersio JF, et al. Baricitinib-induced blockade of interferon gamma receptor and interleukin-6 receptor for the prevention and treatment of graft-versus-host disease. Leukemia. 2018;32(11):2483–94.
Betts BC, Abdel-Wahab O, Young JW, et al. Janus kinase-2 inhibition induces durable tolerance to alloantigen by human dendritic cell-stimulated T cells yet preserves immunity to recall antigen. Blood. 2011;118(19):5330–9.
Wang SP, Iwata S, Tanaka Y, et al. Tofacitinib, a JAK inhibitor, inhibits human B cell activation in vitro. Ann Rheum Dis. 2014;73(12):2213–5.
Parampalli Yajnanarayana S, Stübig T, Wolf D, et al. JAK1/2 inhibition impairs T cell function in vitro and in patients with myeloproliferative neoplasms. Br J Haematol. 2015;169(6):824–33.
Maeshima K, Yamaoka K, Tanaka Y, et al. The JAK inhibitor tofacitinib regulates synovitis through inhibition of interferon-γ and interleukin-17 production by human CD4+ T cells. Arthritis Rheum. 2012;64(6):1790–8.
Ma HH, Ziegler J, Mapara MY, et al. Sequential activation of inflammatory signaling pathways during graft-versus-host disease (GVHD): early role for STAT1 and STAT3. Cell Immunol. 2011;268(1):37–46.
Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem. 2007;282(28):20059–63.
Choi J, Ziga ED, DiPersio JF, et al. IFNγR signaling mediates alloreactive T-cell trafficking and GVHD. Blood. 2012;120:4093–103.
Pérez-Simón JA. Anti-common γ-chain antibody: one for all in GVHD. Blood. 2015;125(3):424–6.
Spoerl S, Mathew NR, von Bubnoff N, et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood. 2014;123(24):3832–42.
Teshima T. JAK inhibitors: a home run for GVHD patients? Blood. 2014;123(24):3691–3.
Carniti C, Gimondi S, Mariotti J, et al. Pharmacologic inhibition of JAK1/JAK2 signaling reduces experimental murine acute GVHD while preserving GVT effects. Clin Cancer Res. 2015;21(16):3740–9.
Ashami K, DiPersio JF, Choi J. Targeting IFNGR/IL6R or downstream JAK1/JAK2 to control GvHD. Oncotarget. 2018;9(87):35721–2.
Takahashi S, Hashimoto D, Teshima T, et al. Ruxolitinib protects skin stem cells and maintains skin homeostasis in murine graft-versus-host disease. Blood. 2018;131:2074–85.
Cetkovic-Cvrlje M, Roers BA, Zeiser R, et al. Targeting Janus kinase 3 to attenuate the severity of acute graft-versus-host disease across the major histocompatibility barrier in mice. Blood. 2001;98(5):1607–13.
Roskoski R. Janus kinase (JAK) inhibitors in the treatment of inflammatory and neoplastic diseases. Pharmacol Res. 2016;111:784–803.
Verstovsek S, Mesa RA, Kantarjian HM. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799–807.
Harrison C, Kiladjian J, Barosi G, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787–98.
Verstovsek S, Passamonti F, Vannucchi AM, et al. A phase 2 study of ruxolitinib, an oral JAK1 and JAK2 Inhibitor, in patients with advanced polycythemia vera who are refractory or intolerant to hydroxyurea. Cancer. 2014;120(4):513–20.
Vannucchi AM, Kiladijan JJ, Verstovsek S, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med. 2015;372:426–35.
Van Vollenhoven RF, Fleischmann R, Wilkinson B, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med. 2012;367(6):508–19.
van Vollenhoven RF, Fleischmann R, Wilkinson B, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med. 2013;369(3):293.
Serhal L, Edwards CJ. Upadacitinib for the treatment of rheumatoid arthritis. Expert Rev Clin Immunol. 2019;15(1):13–25. https://doi.org/10.1080/1744666X.2019.1544892.
Verstovsek S, Komrokji RS. A comprehensive review of pacritinib in myelofibrosis. Future Oncol. 2015;11(20):2819–30.
Mesa RA, Kiladjian JJ, Gotlib J, et al. SIMPLIFY-1: a phase III randomized trial of momelotinib versus ruxolitinib in janus kinase inhibitor-naïve patients with myelofibrosis. J Clin Oncol. 2017;35(34):3844–50.
Pardanani A, Gotlib JR, Tefferi A, et al. Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J Clin Oncol. 2011;29(7):789–96.
Verstovsek S, Tam CS, Shah NP, et al. Phase I evaluation of XL019, an oral, potent, and selective JAK2 inhibitor. Leukemia Res. 2014;38(3):316–22.
Labetoulle R, Paul S, Roblin X. Filgotinib for the treatment of Crohn’s disease. Expert Opin Investig Drugs. 2018;27(3):295–300.
Zeiser R, Burchert A, Von Bubnoff N, et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia. 2015;29(10):2062–8.
Assouan D, Lebon D, Charbonnier A, et al. Ruxolitinib as a promising treatment for corticosteroid-refractory graft-versus-host disease. Br J Haematol. 2018;181(5):687–9. https://doi.org/10.1111/bjh.14679.
Sarmiento Maldonado M, Ramírez Villanueva P, Perez-Simón JA, et al. Compassionate use of ruxolitinib in acute and chronic graft versus host disease refractory both to corticosteroids and extracorporeal photopheresis. Exp Hematol Oncol. 2017;6:32.
Jagasia M, Perales MA, Khoury HJ, et al. (ABSTRACT) results from REACH1, a single-arm phase 2 study of ruxolitinib in combination with corticosteroids for the treatment of steroid-refractory acute graft-vs-host disease. Blood. 2018;132(Suppl 1):601.
Jagasia M, Haris A, Perales MA, et al. (ABSTRACT) ruxolitinib in combination with corticosteroids for the treatment of steroid-refractory acute graft-versus-host disease: results from the phase 2 REACH1 trial. Biol Bone Marrow Transplant. 2019;1:1. https://doi.org/10.1016/j.bbmt.2018.12.130.
Khandelwal P, Teusink-Cross A, Myers KC, et al. Ruxolitinib as salvage therapy in steroid-refractory acute graft-versus-host disease in pediatric hematopoietic stem cell transplant patients. Biol Blood Marrow Transplant. 2017;23(7):1122–7.
Schroeder MA, Khoury HJ, DiPersio JF, et al. (ABSTRACT) A phase I trial of Janus kinase (JAK) inhibition with INCB039110 in acute graft-versus-host disease (aGVHD). Blood. 2016;128(22):390.
Mori Y, Ikeda K, Teshima T, et al. Ruxolitinib treatment for GvHD in patients with myelofibrosis. Bone Marrow Transplant. 2016;51(12):1584–7.
Khoury HJ, Kota VK, DiPersio JF, et al. Ruxolitinib as sparing agent for steroid-dependent chronic graft-versus-host disease (cGVHD). Biol Blood Marrow Transplant. 2017;23(3):S373.
von Bubnoff N, Ihorst G, Zeiser R, et al. Ruxolitinib in GvHD (RIG) study: a multicenter, randomized phase 2 trial to determine the response rate of Ruxolitinib and best available treatment (BAT) versus BAT in steroid-refractory acute graft-versus-host disease (aGvHD) (NCT02396628). BMC Cancer. 2018;18(1):1132.
Jagasia M, Zeiser R, von Bubnoff N, et al. Ruxolitinib for the treatment of patients with steroid-refractory GVHD: an introduction to the REACH trials. Immunotherapy. 2018;10(5):391–402.
Kröger N, Shahnaz Syed Abd Kadir S, Wolschke C, et al. Peritransplantation ruxolitinib prevents acute graft-versus-host disease in patients with myelofibrosis undergoing allogenic stem cell transplantation. Biol Blood Marrow Transplant. 2018;24(10):2152–6.
Vainchenker W, Constantinescu SN. JAK/STAT signaling in hematological malignancies. Oncogene. 2013;32(21):2601–13.
Janson D, Ayuk FA, Kroeger N. (ABSTRACT). Ruxolitinib for myelofibrosis patients relapsing after allogeneic hematopoietic transplantation. Blood. 2016;128(22):1948.
Verstovsek S, Mesa RA, Kantarjian H, COMFORT-I Investigators, et al. Long-term treatment with ruxolitinib for patients with myelofibrosis: 5-year update from the randomized, double-blind, placebo-controlled, phase 3 COMFORT-I trial. J Hematol Oncol. 2017;10(1):55.
Wollenhaupt J, Silverfield J, Riese RJ, et al. Safety and efficacy of tofacitinib, an oral Janus kinase inhibitor, for the treatment of rheumatoid arthritis in open-label, longterm extension studies. J Rheumatol. 2014;41(5):837–52.
Verden A, Dimbil M, Hoffman KB, et al. Analysis of spontaneous postmarket case reports submitted to the FDA regarding thromboembolic adverse events and JAK inhibitors. Drug Saf. 2018;41(4):357–61.
Desai RJ, Pawar A, Kim SC, et al. Comparative risk of venous thromboembolism in rheumatoid arthritis patients receiving tofacitinib versus those receiving tumor necrosis factor inhibitors: an observational cohort study. Arthritis Rheumatol. 2019;71(6):892–900.
Lussana F, Cattaneo M, Squizzato A, et al. Ruxolitinib-associated infections: a systematic review and meta-analysis. Am J Hematol. 2018;93(3):339–47.
Shahnaz Syed Abd Kadir S, Christopeit M, Kroeger N, et al. Impact of ruxolitinib pretreatment on outcomes after allogeneic stem cell transplantation in patients with myelofibrosis. Eur J Haematol. 2018;101(3):305–17.
Heine A, Brossart P, Wolf D. Ruxolitinib is a potent immunosuppressive compound: is it time for anti-infective prophylaxis? Blood. 2013;122(23):3843–4.
Choi J, Cooper ML, DiPersio JF, et al. Pharmacologic blockade of JAK1/JAK2 reduces GvHD and preserves the graft-versus-leukemia effect. PLoS One. 2014;9(10):e109799.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
N.K. received research grants and honoraria for lectures from Novartis. D.M. has no conflicts of interest and nothing to disclose.
Funding
No funding to prepare and write this manuscript was received
Human and animal rights statement
No animal research was done to prepare this manuscript
Rights and permissions
About this article
Cite this article
Mannina, D., Kröger, N. Janus Kinase Inhibition for Graft-Versus-Host Disease: Current Status and Future Prospects. Drugs 79, 1499–1509 (2019). https://doi.org/10.1007/s40265-019-01174-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-019-01174-1