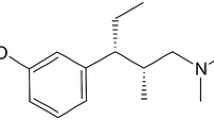
Abstract
Tapentadol prolonged release (tapentadol PR) [Palexia® SR in EU] is a long-acting tablet formulation of the strong central analgesic tapentadol, which acts as both a μ-opioid receptor (MOR) agonist and a noradrenaline reuptake inhibitor. Tapentadol PR is approved for chronic pain in various countries, with its EU indication (severe chronic pain manageable only with opioid analgesics) being the focus here. Well-designed trials and clinical practice data support tapentadol PR use in this setting. Short term, tapentadol PR was an effective and generally well tolerated analgesic for moderate to severe pain of varying aetiologies, including neuropathic pain. It provided analgesia at least as good as that of conventional strong opioids and appeared more favourable in terms of gastrointestinal tolerability, likely due to less potent MOR binding. Severe back pain with a neuropathic component responded well to moderate-dose tapentadol PR in some patients, while for others, an increase to the maximum recommended tapentadol PR dosage provided analgesia at least as good as that of moderate-dose tapentadol PR plus pregabalin and appeared to have some CNS tolerability benefits. Data also support the use of tapentadol PR in opioid rotation, including when conventional opioids are intolerable. Longer-term data in musculoskeletal pain conditions indicate continued benefit over up to 2 years’ treatment with tapentadol PR with no evidence of tolerance. Thus, tapentadol PR is a useful option for the management of severe chronic pain.
Similar content being viewed by others
Acts via μ-opioid receptor agonism and noradrenaline reuptake inhibition |
Reduces moderate to severe chronic pain of varying aetiologies, including neuropathic pain |
At least as effective as conventional strong opioids, but more favourable GI tolerability |
Effective and generally well tolerated over up to 2 years’ therapy, without evidence of tolerance |
1 Introduction
Chronic pain can be debilitating [1] and is often related to a prior injury, disease or surgery with transformation into a chronic state through changes in nervous system/sensory processing [1,2,3,4]. The pain is usually nociceptive, neuropathic or a mixture of the two [5], although its complexity, varying underlying neurobiology and non-linear trajectory make it challenging to manage [6, 7]. The WHO analgesic ladder recommends increasingly strong analgesics for increasing pain intensity, with conventional strong opioids (e.g. morphine, oxycodone) reserved for moderate to severe pain [8]. However, it does not account for underlying pain pathway(s)/mechanism(s), which can be limiting. For instance, neuropathic pain is usually less responsive to opioids than nociceptive pain [9]; exactly why is not completely understood [9], although given conventional opioids exert analgesic effects largely via μ-opioid receptor (MOR) agonism [10, 11], one possibility may be downregulation/desensitization of MORs in certain CNS regions [9].
Conventional opioids are also limited by gastrointestinal (GI) adverse events (AEs), abuse/misuse, addiction and tolerance [12], which can make achieving and maintaining the balance between analgesia and safety difficult. One way to improve this balance is for opioids to be used with analgesics that act via different mechanisms, with those that provide synergistic analgesia (without additional tolerability issues) being particularly beneficial [10]. Such synergism is evident between opioidergic and noradrenergic drugs, the latter of which are particularly effective in alleviating chronic neuropathic pain [10]. A drug combining these two modes of action may therefore provide broad analgesic effects [10], with tapentadol being one such agent. Immediate-release (IR) and prolonged-release (PR) formulations of tapentadol are available in various countries for pain management. This article reviews data relevant to the use of tapentadol PR tablets (Palexia® SR in EU) in the EU-approved indication of severe chronic pain in adults which can be adequately managed only with opioids.
2 Pharmalogical Properties
Tapentadol acts as both a MOR agonist and a noradrenaline reuptake inhibitor (NRI) [13]. The MOR binding affinity of the drug was considerably lower than that of morphine in vitro, although its analgesic potency in animal models was generally only two- to three-times lower than that of morphine [13], highlighting the contribution of NRI activity to its analgesic effect. The opioid and noradrenergic mechanisms of tapentadol were synergistic in some animal analgesia models [14], although the relative contribution of each may vary depending on the pain type [15]. Consistent with its dual mechanism, tapentadol displayed analgesic effects in various preclinical models of pain [13] and was likewise effective in relieving various forms of chronic pain in clinical trials (Sect. 3). Tapentadol’s major metabolite (tapentadol-O-glucuronide) is not active at opioid receptors, synaptosomal reuptake systems or other bindings sites [13].
Tapentadol IR had no clinically relevant impact on ECG parameters in healthy volunteers [16]. Moreover, tapentadol PR had no clinically meaningful impact on heart rate or blood pressure in hypertensive patients with moderate to severe chronic musculoskeletal pain (n = 1464) in a pooled post hoc analysis [17] of three 15-week phase 3 trials [18,19,20] (Sect. 3.1.1); pooled data (n = 4091) [21] from these studies plus a 1-year safety trial (Sect. 3.1.3) [22] in the same setting were consistent with these findings.
Tapentadol reaches maximum serum concentrations 3–6 h after PR tablet administration [23, 24] and, when the tablets are taken twice daily, steady-state levels are attained on day 2 and the accumulation ratio is ≈ 1.5 [23]. Tapentadol PR tablets can be taken without regard to food [23]. Tapentadol has a volume of distribution of 540 L after intravenous administration [23] and is ≈ 20% bound to serum proteins [23]. The drug is extensively metabolized, predominantly via glucuronidation, with UGT1A6, UGT1A9 and UGT2B7 being the main enzymes involved [23]. The drug is also metabolized by CYP2C9 and CYP2C19 or CYP2D6. Excretion of tapentadol and its metabolites is mainly (99%) renal [23]; the apparent oral clearance of the drug after PR tablet administration is 257 L/h (according to population pharmacokinetic modelling) [25] and its average terminal half-life is 5–6 h [23].
Tapentadol PR should be used cautiously (starting at lowest dosage of 50 mg once daily) in moderate hepatic impairment and (given a lack of data) is not recommended in patients with severe renal or hepatic impairment [23]. Elderly patients usually do not require tapentadol PR dosage adjustment, although the dosage should be selected carefully [23].
2.1 Drug Interactions
Tapentadol PR requires care in combination with mixed MOR agonists/antagonists or partial MOR agonists [23]. If tapentadol PR is necessary in buprenorphine recipients, temporary discontinuation of buprenorphine is an option; if taken with buprenorphine, higher tapentadol PR doses may be required, necessitating monitoring for AEs such as respiratory depression (Sect. 4). The respiratory depression risk may be enhanced if tapentadol PR is taken with respiratory depressants, and its sedative effects may be enhanced by CNS depressants; if using tapentadol PR with such agents, consider dosage reductions. Tapentadol PR is contraindicated in patients acutely intoxicated with alcohol, hypnotics, centrally-acting analgesics or psychotropic agents [23].
Clinically relevant glucuronidation-mediated drug interactions are unlikely with tapentadol PR [23]. However, exposure to tapentadol may increase if tapentadol PR is taken with drugs that strongly inhibit UGT isoenzymes involved in its metabolism. Caution is advised if stopping or starting concomitant strong enzyme inducers [23].
Tapentadol PR and monoamine oxidase inhibitors (MAOIs) may have additive effects on noradrenaline at the synapses; thus, tapentadol PR should be avoided during, or within 14 days of stopping, MAOI therapy [23]. Serotonin syndrome has occurred with tapentadol in serotoninergic drug recipients, although symptoms usually improve with serotoninergic agent discontinuation [23]. As tapentadol does not induce or inhibit CYP enzymes in vitro, CYP-mediated drug interactions are unlikely to be clinically relevant with tapentadol PR [23]. Low serum protein binding also makes drug interactions unlikely to occur via protein binding-site displacement [23].
3 Therapeutic Efficacy
This section focuses on the efficacy of tapentadol PR in managing moderate to severe pain, as evaluated in large (n ≥ 250) comparative trials; smaller studies/analyses assessing opioid rotation and real-world data are also discussed. Pain intensity was assessed on an 11-point numerical rating scale (NRS) [0 = no pain; 10 = worse pain imaginable].
3.1 Non-Malignant Musculoskeletal Pain
3.1.1 Versus Placebo
Tapentadol PR was compared with placebo in adults with moderate to severe non-malignant musculoskeletal pain in three 15-week, randomized, double-blind, phase 3 trials [18,19,20]. Each study had a 3-week titration period and a 12-week maintenance period and included oxycodone CR as a reference drug for assay sensitivity (Table 1). However, as one of the trials [18] did not demonstrate significant benefit with oxycodone CR over placebo for primary endpoint measures (Table 1), it was considered to have failed and is not discussed further, except as part of pooled analyses. The two positive studies enrolled adults with chronic lower back pain [19] or osteoarthritic knee pain [20] requiring analgesics (opioid or non-opioid) for the last ≥ 3 months. Patients were dissatisfied with their current analgesia and had an average pain intensity score of ≥ 5 after analgesia washout. Paracetamol was permitted during maintenance.
Tapentadol PR relieved moderate to severe chronic musculoskeletal pain in these studies [19, 20]. Compared with placebo, tapentadol PR significantly (p < 0.05) reduced the average pain intensity both at week 12 of maintenance and throughout the entire maintenance period (primary endpoints; Table 1) [19, 20], regardless of the baseline pain intensity [19]. The proportion of patients with clinically relevant improvements (≥ 30 or ≥ 50%) in pain intensity at week 12 of maintenance was also generally significantly greater with tapentadol PR (Table 1). Tapentadol PR was also associated with significant (p < 0.05 vs. placebo) improvements in health-related quality of life (HR-QOL) and function, as measured by instruments such as the Western Ontario and McMaster Universities Index of Osteoarthritis questionnaire [20], Patient Global Impression of Change (PGIC) scale [19, 20], EuroQol-5 Dimension (EQ-5D) health status index [19, 20] and Short Form-36 Health Survey (SF-36) [19, 20].
These two studies [19, 20] are supported by a pooled analysis [26] of all three phase 3 trials [18,19,20] (see Table 1 for key outcomes). Moreover, in a post hoc pooled subgroup analysis of these three studies, tapentadol PR, unlike oxycodone CR, provided significant (p < 0.01) pain relief versus placebo in elderly patients (aged ≥ 75 years; n = 210), as measured by the least-squares mean (LSM) change from baseline to week 15 in pain intensity [27]. Similarly, when pooled data from two of the trials [18, 20] were assessed retrospectively by age (≥ 75, ≥ 65 or < 65 years; n = 176, 653 and 1357, respectively), tapentadol PR provided analgesic benefit over placebo (based on 95% CIs for between-group difference) regardless of age group, as indicated by one or both of the primary endpoints (LSM change from baseline in average pain intensity at week 12 of maintenance or over the entire maintenance period) [28]. Corresponding analgesic benefit was not evident with oxycodone CR versus placebo in any of the age groups using these measures [28].
3.1.2 Versus Active Regimens
Tapentadol PR was compared with oxycodone/naloxone PR [29] and tapentadol PR plus pregabalin [30] in adults with severe chronic lower back pain with a neuropathic component in two phase 3b [30] or 3b/4 [29] noninferiority trials of open-label [29] or double-blind [30] design. The pain must have lasted for ≥ 3 months, require a strong (i.e. WHO step III) analgesic and be rated as ‘positive’ or ‘unclear’ for neuropathic pain on the painDETECT questionnaire. Tapentadol PR has also been compared with oxycodone CR in patients with moderate to severe chronic musculoskeletal pain in pooled analyses of the phase 3 trials in Sect. 3.1.1.
3.1.2.1 Oxycodone/Naloxone PR
Patients needed an average pain intensity score of ≥ 6 at randomization (which occurred after washout of centrally-acting analgesics or co-analgesics) [29]. After randomization to tapentadol PR or oxycodone/naloxone PR, patients entered a 3-week titration period, with those who achieved acceptable/satisfactory pain relief (i.e. pain intensity score ≤ 4 or ≤ 5) and tolerability then able to continue their allocated study drug at a stable dosage for a further 9 weeks. Patients who did not reach this target discontinued the trial, with those in the oxycodone/naloxone PR group also having the option of switching to tapentadol PR in a ‘pick-up’ arm.
Over 12 weeks, tapentadol PR was more effective than oxycodone/naloxone PR in alleviating severe chronic lower back pain with a neuropathic component, providing significantly greater reductions in average pain intensity (primary endpoint), the intensity of pain radiating to the leg, and neuropathic pain measures, including painDETECT total score and Neuropathic Pain Symptom Inventory (NPSI) overall feeling score (Table 2) [29]. There were also significantly (p < 0.001) more tapentadol PR than oxycodone/naloxone PR recipients with a ‘much improved’ or ‘very much improved’ overall health status, as rated by patients (PGIC) and investigators (clinician global impression of change; CGIC) at final evaluation [29]. Patients in the pick-up arm who received tapentadol PR after switching from oxycodone/naloxone PR also had significant improvements in pain intensity and neuropathic pain measures (Table 2), including from the pick-up baseline to final evaluation [29].
3.1.2.2 Tapentadol PR plus Pregabalin
Patients needed an average pain intensity score of ≥ 6 at baseline (if on a WHO step I analgesic or not taking analgesics regularly) or a ≥ 1-point increase in the score after washing out WHO step II or III analgesics or centrally-acting co-analgesics [30]. Eligible patients were then titrated to moderate-dose tapentadol PR (300 mg/day) in a 3-week open-label period. Those whose pain intensity score declined by ≥ 1 point during titration but still averaged ≥ 4 were subsequently randomized to double-blind treatment with high-dose tapentadol PR (500 mg/day target) or moderate-dose tapentadol PR plus pregabalin (300 mg/day target) for 8 weeks. Patients who did not qualify for randomization (i.e. had satisfactory pain relief) could continue tapentadol PR 300 mg/day in an 8-week open-label continuation arm; patients who discontinued double-blind treatment for AEs possibly related to treatment could receive a lower tapentadol PR dosage (300 or 400 mg/day) in an open-label pick-up arm. Paracetamol was permitted for non-lower back pain in the comparative and continuation arms.
For severe chronic lower back pain with a neuropathic component, high-dose tapentadol PR provided analgesia noninferior to that of moderate-dose tapentadol PR plus pregabalin, as measured by the change from randomization to final evaluation in average pain intensity (primary endpoint; Table 2) [30]. During this 8-week period, each regimen also significantly (p < 0.0001) improved the intensity of pain radiating to the leg (Table 2) and measures of neuropathic pain, including painDETECT and NPSI total scores (Table 2), with no marked differences between the groups. Most tapentadol PR and tapentadol PR plus pregabalin recipients had an improved overall health status (i.e. ‘minimally’, ‘much’ or ‘very much’ improved) at final evaluation, as assessed on PGIC (81 and 82%) and CGIC (83 and 83%) scales [30]. In addition, most evaluated HR-QOL measures significantly (p < 0.05) improved with tapentadol PR [including six of eight SF-12 subscale scores, the SF-12 physical health (but not mental health) composite score and the EQ-5D health status index] and all significantly (p < 0.05) improved with tapentadol PR plus pregabalin. Both treatment groups also reported significant (p < 0.05) improvements in anxiety and depression (on the Hospital Anxiety and Depression Scale; HADS) and mean duration of sleep [30].
Some patients achieved adequate pain relief with moderate-dose tapentadol PR alone (see continuation arm data; Table 2). These patients had a mean pain duration at enrolment of 7.9 years (vs. 9.4 and 8.7 years in the high-dose tapentadol PR and moderate-dose tapentadol PR plus pregabalin groups) [30].
3.1.2.3 Oxycodone CR
Across pooled analyses of two (knee osteoarthritis pain [18, 20]; n = 2010) [31] or three (knee osteoarthritis and lower back pain [18,19,20]; n = 2968) [26] phase 3 studies (Sect. 3.1.1), tapentadol PR was at least as effective as oxycodone CR in relieving moderate to severe musculoskeletal pain and had more HR-QOL/health status benefits. For instance, compared with oxycodone CR in the largest analysis [26], tapentadol PR was superior in reducing the average pain intensity over the entire maintenance period (primary endpoint) [32], noninferior in reducing the average pain intensity at week 12 of maintenance (primary endpoint) [26], and significantly more tapentadol PR recipients achieved a ≥ 30 or ≥ 50% reduction in pain intensity from baseline to week 12 [26] (Table 1). Tapentadol PR was also generally associated with significantly (p < 0.05) more favourable changes in HR-QOL (SF-36) and health status (EQ-5D health status index and PGIC score distribution) versus oxycodone CR [26].
When pooled data for the elderly patients in these three studies were assessed post hoc, the analgesic benefit of tapentadol PR did not significantly differ from that of oxycodone CR, as measured by the LSM change from baseline to week 15 in pain intensity [27]. The relative analgesic efficacy of these agents in this and other patient age groups (≥ 75, ≥ 65 or < 65 years) in a retrospective analysis [28] of two of the studies [18, 20] (pooled) supported these findings.
Some of the differences observed between tapentadol PR and oxycodone CR, particularly for HR-QOL, may reflect the less favourable tolerability profile of oxycodone CR (Sect. 4.1). Indeed, composite outcomes of pain relief and tolerability were achieved by significantly (p < 0.001) more tapentadol PR than oxycodone CR recipients in a post hoc meta-analysis of the three phase 3 studies [33].
3.1.3 Longer-Term Therapy
Longer-term data are from two open-label phase 3 studies (designed primarily to assess safety) in adults with moderate to severe chronic musculoskeletal pain (knee/hip osteoarthritis or lower back) [22, 34]. The first was an oxycodone CR-controlled trial in patients with ≥ 3 months of pain, dissatisfaction with their current analgesia and a pain intensity score (after analgesic washout) of ≥ 4 [22]. Patients eligible for this 1-year study were randomized to adjustable twice-daily doses of tapentadol PR (100–250 mg) or oxycodone CR (20–50 mg) for 52 weeks [22]. The second trial [34] was a single-arm extension in patients who had completed the 1-year study [22], one of two 15-week trials (Sect. 3.1.1) [19, 20] or a 7-week dose-conversion study [35]. The extension consisted of ≤ 4 weeks’ titration (to optimal tapentadol PR dosage; however, patients who had received tapentadol PR in the 1-year study continued their dosage without titration) and a maintenance phase of ≤ 48 weeks (optimal dosage continued) [34].
Analgesic efficacy was evident over up to 2 years’ tapentadol PR therapy [22, 34]. In the comparative 1-year trial [22], tapentadol PR (n = 876) and oxycodone CR (n = 219) recipients had mean pain intensity scores of 4.4 and 4.5 at endpoint versus 7.6 each at baseline, and the most common PGIC health status rating in each of the groups at treatment end was ‘much improved’ (36 vs. 33% of patients). Patients who had received tapentadol PR in the 1-year trial and continued treatment in the 1-year extension (n = 249) maintained their improvements in pain intensity (mean pain intensity score was 3.43 at extension baseline vs. 3.67 at extension end) with relatively stable daily doses of tapentadol PR, giving no indication of tolerance development to the analgesic effect [34]. Similar findings were reported for the overall extension population (n = 1149) [34].
3.2 Painful Diabetic Peripheral Neuropathy
Tapentadol PR was evaluated in adults with moderate to severe chronic pain associated with diabetic peripheral neuropathy (DPN) in two placebo-controlled phase 3 trials with similar enriched-enrollment randomized-withdrawal designs [36, 37]. Patients must have had ≥ 6 months of painful DPN, for which they had used analgesics for ≥ 3 months but were unsatisfied with their current regimen. After analgesic washout, patients with an average pain intensity score of ≥ 5 entered a 3-week open-label titration period to determine the optimal tapentadol PR dosage. Those who experienced a ≥ 1-point improvement in average pain intensity were then randomized to continue their optimal tapentadol PR dosage or switch to placebo for the 12-week double-blind maintenance period. Trials allowed rescue analgesia [37] or additional analgesia (paracetamol during titration; tapentadol PR 25–50 mg during maintenance) [36].
Tapentadol PR was effective in managing DPN-associated pain. The drug provided significant benefit over placebo in maintaining the clinically important improvement in pain achieved with tapentadol PR during titration, as measured by the change in average pain intensity from the start to the end of the 12-week maintenance period (primary endpoint; Table 3) [36, 37]. Where specified [36], the change with tapentadol PR was of similar magnitude regardless of patient sex, age or opioid experience. Moreover, significantly more tapentadol PR than placebo recipients achieved ≥ 30 or ≥ 50% improvements in pain intensity from pre-titration to week 12 of maintenance (Table 3) [36, 37]. The drug was also associated with more favourable (p ≤ 0.015) changes than placebo in NPSI-measured neuropathic pain [37].
PGIC score distribution indicated significant (p < 0.001 vs. placebo) overall improvement in health status with tapentedol PR [37], with 1.7-fold (64 vs. 38%; p < 0.001) [36] or 1.5-fold (66 vs. 45%) [37] more tapentadol PR than placebo recipients reporting a status of ‘very much’ or ‘much’ improved at maintenance end. Changes in some HR-QOL measures (including EQ-5D health status index, two of eight SF-36 domain scores and SF-36 physical component summary score) also significantly (p ≤ 0.004) favoured tapentadol PR over placebo during maintenance [37].
A post hoc pooled analysis [38] of these trials supported the individual study findings (Table 3).
3.3 Cancer-Related Pain
Two phase 3 trials (each of which used morphine IR as rescue therapy) compared tapentadol PR with placebo [39], morphine CR [39] or oxycodone CR [40] in adults with moderate to severe, chronic cancer-related pain. The studies enrolled patients from Europe who were opioid naive or dissatisfied with opioids and had a pain intensity score of ≥ 5 (on current analgesia) [39] or patients from Japan/Korea who were dissatisfied with their analgesia, required opioids and had a pain intensity score of ≥ 4 (without taking opioids for pain in last 28 days) [40].
One of the studies [39] randomized patients to twice-daily tapentadol PR 100–250 mg (n = 338) or morphine CR 40–100 mg (n = 158) in a 2-week double-blind titration period for dosage optimization. Patients meeting stabilization criteria (i.e. mean pain intensity score < 5 and morphine IR mean of ≤ 20 mg/day) could enter the 4-week double-blind maintenance phase. For this period, the patients on tapentadol PR were re-randomized to placebo (n = 112) or continued the optimal dosage of tapentadol PR (n = 106) [i.e. randomized-withdrawal], while those on morphine CR continued the drug at the optimal dosage after sham re-randomization (n = 109); all comparisons with morphine CR during maintenance were descriptive. The other study [40] randomized eligible patients to receive twice-daily tapentadol PR 25–200 mg (n = 168) or oxycodone CR 5–40 mg (n = 172) in a double-blind fashion for 4 weeks, which included titration to, and then maintenance of, an optimal dosage.
Tapentadol PR relieved pain in this setting, with the adjusted estimated rate of response at the end of the 4-week maintenance period in the full analysis set (FAS) being significantly (p = 0.02) greater with tapentadol PR than with placebo (64.3 vs. 47.1%; odds ratio 2.02; 95% CI 1.12–3.65) [primary endpoint analysis, with response being completion of maintenance, plus a mean pain intensity score < 5 and average ≤ 20 mg/day morphine IR rescue therapy during maintenance] [39]. Observed responder rates during maintenance supported these findings and indicated benefit in both the tapentadol PR (n = 105 FAS) and morphine CR (n = 109) groups versus placebo (n = 111), regardless of whether the pain was nociceptive, neuropathic or visceral (51–74 and 55–70 vs. 38–54% of placebo recipients) [39].
With regard to formal active comparisons, tapentadol PR provided analgesia at least as good as that of oxycodone CR [40] and morphine CR [39]. Tapentadol PR (n = 126 per protocol set) was noninferior to oxycodone CR (n = 139) for the mean change in average pain intensity from baseline (5.4 and 5.3) to the last 3 days of the 4-week treatment period (− 2.69 vs. − 2.57; 95% CI − 0.51, 0.38) [primary endpoint, with pain intensity rated once daily and averaged over last 24 h], regardless of patient age, baseline pain intensity or country (Korea or Japan) [40]. The tapentadol PR and oxycodone CR groups also did not markedly differ with regard to the proportion of patients who achieved a ≥ 30% (64 vs. 59%) or ≥ 50% (50 vs. 42%) improvement in pain intensity, considered their overall clinical status as improved (‘very much’, ‘much’ or ‘minimally’) on the PGIC (89.7 vs. 82.7%) or used morphine IR rescue therapy (74.6 vs. 74.1%) [40].
Similarly, at the end of titration in the other trial [39], tapentadol PR met the prespecified criteria for noninferiority to morphine CR in terms of the observed rate of response (defined as titration completion, plus a mean pain intensity score of < 5 and average use of ≤ 20 mg/day morphine IR, during last 3 days of titration). The response rate was 76.0% with tapentadol PR (n = 229 per protocol) and 83.0% with morphine CR (n = 100), with noninferiority established at a dose ratio of 2.5: 1. In the respective groups, 72 and 58% of patients took rescue medication during titration.
3.4 Switching from Other Opioids
In open-label phase 3 [41] or 3b [42, 43] trials, switching to tapentadol PR (50–250 mg twice daily) maintained analgesia in patients with severe chronic pain of the lower back (n = 123) [42] or knee (n = 62) [43] or moderate to severe chronic cancer pain (n = 100) [41] already responding to strong conventional opioids (average pain intensity score ≤ 5 [42, 43] or < 4 [41]), but wishing to switch because of poor tolerability [42, 43]. For instance, in the largest trial (which comprised a 5-week tapentadol PR titration/stabilization period and a 7-week maintenance period), 80.9% of patients had the same or lower pain intensity score at week 6 versus week − 1 (primary endpoint), indicating noninferior analgesic efficacy of tapentadol PR versus prior strong opioid therapy [42]. At weeks 6, 8 and 12, mean pain intensity scores were significantly (p < 0.0001) reduced from baseline and 79–87% of patients had improved overall health status, as rated by patients (PGIC) and investigators (CGIC). There were also significant (p < 0.05) improvements in HR-QOL, anxiety and depression at one or more of these timepoints, as measured by SF-36, EQ-5D and HADS. The equianalgesic ratios of tapentadol PR to oxycodone PR (4.3:1), morphine PR (2.9:1) and other strong opioid formulations were consistent with those in other phase 3/3b trials [42].
Patients with moderate to severe chronic cancer pain on the weak opioid tramadol may also be switched directly to tapentadol PR, according to post hoc analysis [44] of another phase 3 trial [39] (Sect. 3.3), as the response rate in this subgroup (n = 129) after 2 weeks of titration was 69.8% versus 63.9% in the overall tapentadol PR population (n = 338).
3.5 Real-World Data
The efficacy of tapentadol PR in relieving severe chronic pain in the real-world setting has been seen in various studies, with a large fully published analysis (n = 3134) [45] discussed here. Most patients in this prospective, non-interventional study had back pain (82%), mixed pain components (84%) and a pain duration of > 1 year (62%). Patients were generally receiving long-term analgesia often comprising various different analgesics (83% were taking non-opioids, 53% weak opioids and 43% strong opioids), and were switching to tapentadol PR most commonly because their medication(s) provided insufficient pain relief. Over a period of ≈ 3 months, tapentadol PR reduced the mean intensity of pain (over last 3 days) from 7.0 at baseline to 3.1 (descriptive p ≤ 0.001), with most patients (72%) achieving a ≥ 50% reduction in pain by the period’s end. HR-QOL, social activity, self-sufficiency, libido and sleep quality also improved (descriptive p ≤ 0.001 vs. baseline for each).
4 Tolerability
Tapentadol PR use for up to 2 years was generally well tolerated in the clinical trials, pooled analyses and clinical practice study discussed in Sect. 3, with its tolerability profile being similar regardless of the pain type. Notably, patients switched directly from strong conventional opioids to tapentadol PR because of poor tolerability had a decline in the incidence of AEs for which the switch was desired, the most common of which were constipation [36% of 125 patients at week − 1 (i.e. on prior strong opioid)] vs. 18% of 93 patients at week 12 (i.e. on tapentadol PR)] and nausea (20 vs. 14%) [42]. Tapentadol PR was also generally well tolerated in patients switched from the weak opioid tramadol [44].
As with other opioids, respiratory depression may occur with tapentadol PR (at high doses or in patients sensitive to MOR agonists) and, being a MOR agonist, it may cause spasm of the hepatopancreatic sphincter [23]. Tapentadol PR requires caution in patients with impaired respiratory function or biliary tract disease and is contraindicated in patients with significant respiratory depression, acute/severe bronchial asthma or hypercapnia or with paralytic ileus [23].
4.1 Versus Oxycodone CR
In the large pooled analysis (of four phase 3 trials of 15 weeks’ or 1 year duration) in patients with chronic lower back or osteoarthritic knee pain (Sect. 2) [21], treatment-emergent AEs (TEAEs) occurred in most tapentadol PR and oxycodone CR recipients (79.0 vs. 86.6%; placebo incidence was 58.7%), although were rarely serious (< 4% of patients in each group); however, almost half as many tapentadol PR than oxycodone CR recipients discontinued treatment because of TEAEs (20.1 vs. 38.6%; placebo incidence was 6.3%). The most common TEAEs with each treatment were GI and nervous system disorders, with the largest between-group difference in incidence being for the former and favouring tapentadol PR (Fig. 1), although both were usually mild or moderate, irrespective of the treatment group [21]. Tapentadol PR also had more favourable GI tolerability than oxycodone CR in elderly [27, 28] and younger [28] patients in pooled subgroup analyses.
Adverse event profile of tapentadol prolonged release (system organ class and individual); a pooled analysis of phase 3 trials of ≤ 1 years’ duration [21]. bid twice daily, GI gastrointestinal, NS nervous system, OXY CR oxycodone controlled release, PL placebo, TAP PR tapentadol prolonged release, TEAEs treatment-emergent adverse events
The most common individual TEAEs were similar in nature with tapentadol PR and oxycodone CR in the large pooled analysis, although most (including constipation, nausea, dizziness, somnolence, vomiting and pruritus) occurred with numerically lower incidence with tapentadol PR (Fig. 1) [21]. In patients with treatment-emergent constipation, the symptoms appeared to be less severe with tapentadol PR than with oxycodone CR, as measured by the mean change from baseline in the Patient Assessment of Constipation Symptoms (PAC-SYM) questionnaire total score (0.3 vs. 0.7; placebo change was 0.3, with increases indicating worsening) and individual subscale scores [21]. The TEAEs that most often led to discontinuation of tapentadol PR were dizziness, nausea and vomiting, although the incidence of such discontinuations was up to four times lower than with oxycodone CR (2.8–3.6 vs. 8.7–14.0%; placebo range was 0.7–0.8%) [21].
4.2 Versus Other Active Comparators
The overall GI tolerability of tapentadol PR was more favourable than that of some conventional strong opioid regimens in phase 3 or 3b/4 studies.
Compared with oxycodone/naloxone PR in patients with severe lower back pain with a neuropathic component [46], tapentadol PR was noninferior in terms of bowel dysfunction symptom severity, as measured by the mean change from baseline to final evaluation in SYM-PAC total score (0.07 vs. 0.14; 97.5% exact repeated CI for between-group difference − 0.26 to 0.12) [primary safety endpoint] [46]. However, this outcome may have been impacted by the fact that more (p < 0.001) oxycodone/naloxone PR than tapentadol PR recipients discontinued treatment during the 3-week titration (40 vs. 19%) and 12-week overall treatment (42 vs. 22%) periods and thus did not reach maximal dosage. There was a significantly (p < 0.05) lower incidence of constipation and vomiting with tapentadol PR than with oxycodone/naloxone PR during each of these periods (e.g. 15.4 vs. 25.8% and 7.7 vs. 16.4% in overall treatment period). Tapentadol PR was also significantly better than oxycodone/naloxone PR for the combined incidence of nausea, vomiting and/or constipation during titration (32 vs. 46%; exploratory p = 0.03), although between-group differences for other predefined TEAE composites were not significant.
Likewise, when compared with morphine CR over a 2-week titration period in patients with cancer pain, tapentadol PR was associated with a significantly (post hoc p < 0.004) lower incidence of TEAEs (50 vs. 64% of patients) and GI disorders (30 vs. 47%), including nausea (12 vs. 24%), vomiting (5 vs. 16%) and dry mouth (1 vs. 6%), but not constipation (14 vs. 18%); the two treatment groups did not significantly differ in terms of nervous system disorders or general/administration-site conditions [39]. In the 4-week maintenance phase (during which comparisons were descriptive and patients had titrated to dosages they tolerated well), there were less marked differences between the tapentadol PR and morphine CR groups in the incidence of TEAEs and GI disorders. Serious TEAEs occurred in 7% of tapentadol PR recipients during titration (vs. 4% of morphine CR recipients) and 11% during maintenance (vs. 6% of morphine CR and 9% of placebo recipients) and no fatal TEAEs were considered related/likely related to study drug. TEAEs did not often result in tapentadol PR discontinuation during either of these periods (9 vs. 7% of morphine CR recipients during titration; 5 vs. 6% of morphine CR and 5% of placebo recipients during maintenance) [39].
When used to treat severe chronic musculoskeletal pain with a neuropathic component in a phase 3b trial, the tolerability profile of high-dose tapentadol PR was generally similar to that of moderate-dose tapentadol PR plus pregabalin, but was more favourable in terms of some CNS events [30]. With monotherapy, the most common TEAEs were hyperhidrosis (12 vs. 6% of combination recipients) and dizziness (11 vs. 18%), whereas in the combination group they were dizziness (18 vs. 11% of monotherapy recipients) and somnolence (12 vs. 8%); significantly (p = 0.03) fewer monotherapy than combination recipients experienced dizziness and/or somnolence (17 vs. 27%) [post hoc composite] [30].
4.3 Longer-Term Profile
Tolerability data over up to 2 years are from the 1-year single-arm extension discussed in Sect. 3.1.3, the participants of which had previously received ≤ 15 weeks or 1 year of tapentadol PR (n = 358 and 249) or oxycodone CR (n = 199 and 45) or had previously received placebo (n = 303) [34]. Overall, the tolerability profile of tapentadol PR during the extension was generally consistent with that of the large pooled analysis (Sect. 4.1) [21], with the most commonly observed TEAEs being GI disorders (37% of patients) [34]. However, when data were assessed by what patients had received prior to the extension, those who had previously received 1 year of tapentadol PR had the lowest incidence of GI disorders (24 vs. 31–51% across other groups). Among these patients, no individual TEAE had an incidence > 7% during year 2 of treatment, and < 10% of patients had serious TEAEs or discontinued tapentadol PR because of TEAEs over the entire 2 years of therapy [34].
Withdrawal assessment using the Clinical Opiate Withdrawal Scale found patients who abruptly discontinued tapentadol PR in the extension either did not experience opiate withdrawal symptoms within the 2–4 or ≥ 5 days after discontinuing (89 or 91%) or had only mild/moderate withdrawal during these periods (11 or 9%) [34]. Assessment of withdrawal using the Subjective Opiate Withdrawal Scale was consistent with these findings [34].
5 Dosage and Administration
In various European countries, including the UK [23], tapentadol PR 50–250 mg tablets are indicated for the management of severe chronic pain in adults, which can be adequately managed only with opioid analgesics. The tablets should be taken twice daily (every ≈ 12 h) without chewing or dividing. The dosage should be individualized on the basis of pain severity, ability of the patient to be monitored and their prior treatment experience, including the nature, administration route and mean daily dose of prior therapy [23]. Patients currently taking opioids may require a higher starting dosage of tapentadol PR than opioid-naïve patients. The tapentadol PR dosage should be titrated to attain adequate analgesia while minimizing AEs; the total daily dosage should not exceed 500 mg [23]. For patients at increased risk of abuse, misuse, addiction or diversion, the potential for tapentadol PR abuse/addiction should be considered; monitor all recipients carefully for signs of abuse/addiction [23]. Consult local prescribing information for detailed information regarding use in special populations, drug interactions, contraindications and other warnings/precautions.
6 Place of Tapentadol PR in Managing Pain
Prescribing of analgesics has increased dramatically in recent years [4] and the public health harms associated with opioid use (e.g. abuse, overdose and diversion) are now considerable [47], prompting their role in pain management to be re-assessed [4]. Various non-pharmacological therapies and nonopioid agents (e.g. paracetamol, NSAIDs, anticonvulsants, antidepressants) can alleviate chronic pain and are preferred in some guidelines [48]. However, opioids are considered crucial for cancer pain [49], and although their long-term use in chronic noncancer pain generally lacks evidential support, they are considered appropriate for some patients [7, 48, 50], including those for whom more conservative/interventional strategies are ineffective [50] and when the pain/function benefits are expected to outweigh the risks [48].
Tapentadol is a strong analgesic, but unlike strong opioids (e.g. morphine, oxycodone, hydromorphine, fentanyl, buprenorphine, diamorphine and methadone) and other centrally-acting analgesics, tapentadol is a multi-modal drug, acting as both a MOR agonist and an NRI (Sect. 2). The only other opioid with a similar dual mechanism is tramadol, although in contrast to tapentadol, tramadol also has serotonergic properties and its mechanisms reside in different enantiomers rather than a single molecule, diminishing the likelihood of synergism [10].
Consistent with its dual mode of action, tapentadol PR relieved moderate to severe chronic pain of varying aetiologies, including neuropathic pain, in short-term trials, with benefits also evident in functional and HR-QOL measures (Sect. 3). Being able to manage various pain types with a single analgesic could simplify pain management. Moreover, tapentadol PR was at least as good as oxycodone CR and morphine CR in alleviating moderate to severe cancer-related pain and at least as good as oxycodone CR for moderate to severe musculoskeletal pain. However, the drug appeared to be more favourable than these conventional opioids in terms of GI tolerability (Sect. 4), likely due to being less potent at the MOR (Sect. 2), as gut MOR agonism is key in the adverse GI effects of opioids [51]. Indeed, the contribution of tapentadol’s MOR agonism to constipation in clinical trials was 38–41% relative to conventional μ-opioids at equianalgesia (the ‘μ-load’ concept) [11].
To address opioid-induced bowel dysfunction, opioids can be coadministered with an opioid antagonist, such as naloxone. However, tapentadol PR was more effective than oxycodone/naloxone PR in alleviating severe lower back pain with a neuropathic component in a short-term trial (Sect. 3.1.2.1) and its overall GI tolerability profile was more favourable (Sect. 4.2). As patients often discontinue opioid therapy because of GI tolerability issues [51], the more favourable GI tolerability profile of tapentadol PR (vs. this and other conventional opioid regimens discussed earlier) may improve compliance to treatment, although this remains to be formally assessed.
Severe neuropathic (and mixed) pain can be hard to treat with conventional strong opioids alone, as MOR agonism may not adequately address every pain mechanism involved. Combined use of an opioid and anti-convulsant (e.g. gabapentin or pregabalin) is an option, although tolerability can be problematic [7]. A short-term study found this type of pain responded well to moderate-dose tapentadol PR in some patients, while for others, an increase to the maximum recommended tapentadol PR dosage provided analgesia noninferior to that of moderate-dose tapentadol PR plus pregabalin (Sect. 3.1.2.2). Regimen tolerability was generally similar, although tapentadol PR appeared more favourable with regard to some CNS AEs (Sect. 4.2), which are known to limit combination regimen use [7].
If an opioid is ineffective/not tolerated, opioid rotation is an option [7], with data from clinical trials and routine clinical practice supporting the use of tapentadol PR in this setting (Sects. 3.4, 3.5 and 4). When switching between opioids, avoiding potential over- or under-dosing (and thus tolerability or withdrawal issues) is important [32]. For patients switching from one conventional opioid to another, a reduction in the dosage of the next opioid is usually advised [32]. However, when switching from a conventional opioid to tapentadol PR, the fact that tapentadol is not a pure opioid (Sect. 2) must be taken into account when selecting its starting dosage, to minimize the potential for withdrawal from the prior opioid [32]. Thus, the starting dosage of tapentadol PR may need to be higher for opioid-experienced than -naïve patients (Sect. 5). Furthermore, unlike several conventional opioids (e.g. oxycodone, hydrocodone, tramadol and codeine) [7], tapentadol does not undergo CYP-mediated metabolism or have active metabolites (Sect. 2), and may therefore have more predictable pharmacokinetics/analgesia. It also carries a low risk of drug interactions mediated by CYP or protein binding displacement (Sect. 2.1) and may thus be a prudent option for polypharmacy patients (e.g. the elderly).
Robust studies evaluating the long-term benefit of opioids for chronic pain beyond 1 year of treatment are few [48]. However, tapentadol PR provided effective (Sect. 3.1.3) and generally well tolerated (Sect. 4.3) analgesia for moderate to severe musculoskeletal pain over up to 2 years of therapy, with no evidence of tolerance development (Sect. 3.1.3). Withdrawal symptoms (which can be problematic when discontinuing opioids, especially after prolonged therapy [4]) were minimal following abrupt discontinuation of tapentadol PR (Sect. 4.3).
Various measures can be implemented to reduce the inappropriate use of opioids, among which are abuse-deterrent formulations [52], with such formulations being available for tapentadol and various other opioids in certain markets [52]. Although abuse-deterrent opioid formulations are an important step in addressing the manipulation of opioids for non-oral use, their large-scale impact in preventing opioid abuse remains to be determined [52]. As an active pharmaceutical ingredient (not accounting for formulation), tapentadol had lower rates of abuse than most/all (depending on drug availability adjustment) other opioids in a retrospective analysis of abuse liability data from the Researched Abuse, Diversion and Addiction-Related Surveillance system [53]. Abuse potential data specific to the EU formulation of tapentadol PR would be of interest.
To conclude, tapentadol PR is a useful option for patients with severe chronic pain manageable only with opioid analgesics, including those for whom conventional opioids are poorly tolerated.
Data selection sources: EMBASE, MEDLINE and PubMed from 2012 to present. Previous Adis Drug Evaluation published in 2012 was hand-searched for relevant data. Clinical trial registries/databases and websites were also searched for relevant data [searches last updated 25 October 2018]. Records were limited to those in English language. |
Search terms: Tapentadol, Palexia, Nucynta, TAPAL, BN200, CG5503, R331333, JNS024, extended-release. |
Change history
21 March 2019
The article Tapentadol Prolonged Release: A Review in Pain Management, written by Emma D. Deeks, was originally published Online First without open access.
21 March 2019
The article Tapentadol Prolonged Release: A Review in Pain Management, written by Emma D. Deeks, was originally published Online First without open access.
21 March 2019
The article Tapentadol Prolonged Release: A Review in Pain Management, written by Emma D. Deeks, was originally published Online First without open access.
21 March 2019
The article Tapentadol Prolonged Release: A Review in Pain Management, written by Emma D. Deeks, was originally published Online First without open access.
21 March 2019
The article Tapentadol Prolonged Release: A Review in Pain Management, written by Emma D. Deeks, was originally published Online First without open access.
21 March 2019
The article Tapentadol Prolonged Release: A Review in Pain Management, written by Emma D. Deeks, was originally published Online First without open access.
21 March 2019
The article Tapentadol Prolonged Release: A Review in Pain Management, written by Emma D. Deeks, was originally published Online First without open access.
References
Raffaeli W, Arnaudo E. Pain as a disease: an overview. J Pain Res. 2017;10:2003–8.
Renn CL, Dorsey SG. The physiology and processing of pain: a review. AACN Clin Issues. 2005;16(3):277–90.
Ossipov MH, Morimura K, Porreca F. Descending pain modulation and chronification of pain. Curr Opin Support Palliat Care. 2014;8(2):143–51.
British Medical Association. Chronic pain: supporting safer prescribing of analgesics. 2017. http://www.bma.org.uk. Accessed 30 Oct 2018.
Tennant F. Types of chronic pain. 2015. https://www.practicalpainmanagement.com. Accessed 30 Oct 2018.
Ballantyne JC, Kalso E, Stannard C. WHO analgesic ladder: a good concept gone astray. BMJ. 2016;352:i20.
Scottish Intercollegiate Guidelines Network. Management of chronic pain. A national clinical guideline. 2013. http://www.sign.ac.uk. Accessed 30 Oct 2018.
World Health Organization. WHO’s cancer pain ladder for adults. 2015. http://www.who.int. Accessed 30 Oct 2018.
Smith HS. Opioids and neuropathic pain. Pain Physician. 2012;15(3 Suppl):ES93–ES110.
Tzschentke TM, Christoph T, Kogel BY. The mu-opioid receptor agonist/noradrenaline reuptake inhibition (MOR-NRI) concept in analgesia: the case of tapentadol. CNS Drugs. 2014;28(4):319–29.
Raffa RB, Elling C, Tzschentke TM. Does ‘strong analgesic’ equal ‘strong opioid’? Tapentadol and the concept of ‘μ -load’. Adv Ther. 2018. https://doi.org/10.1007/s12325-018-0778-x.
Vadivelu N, Huang Y, Mirante B, et al. Patient considerations in the use of tapentadol for moderate to severe pain. Drug Healthc Patient Saf. 2013;5:151–9.
Tzschentke TM, Christoph T, Kögel B, et al. (-)-(1R,2R)-3-(3-dimethylamino-1-ethyl-2-methyl-propyl)-phenol hydrochloride (Tapentadol HCl): a novel μ-opioid receptor agonist/norepinephrine reuptake inhibitor with broad-spectrum analgesic properties. J Pharmacol Exp Ther. 2007;323(1):265–76.
Schröder W, Tzschentke TM, Terlinden R, et al. Synergistic interaction between the two mechanisms of action of tapentadol in analgesia. J Pharmacol Exp Ther. 2011;337(1):312–20.
Schröder W, Vry JD, Tzschentke TM, et al. Differential contribution of opioid and noradrenergic mechanisms of tapentadol in rat models of nociceptive and neuropathic pain. Eur J Pain. 2010;14(8):814–21.
Oh C, Rengelshausen J, Mangold B, et al. A thorough QT/QTc study of multiple doses of tapentadol immediate release in healthy subjects. Int J Clin Pharmacol Ther. 2010;48(10):678–87.
Biondi DM, Xiang J, Etropolski M, et al. Evaluation of blood pressure and heart rate in patients with hypertension who received tapentadol extended release for chronic pain: a post hoc, pooled data analysis. Clin Drug Investig. 2014;34(8):565–76.
Serrie A, Lange B, Steup A. Tapentadol prolonged-release for moderate-to-severe chronic osteoarthritis knee pain: a double-blind, randomized, placebo- and oxycodone controlled release-controlled study. Curr Med Res Opin. 2017;33(8):1423–32.
Buynak R, Shapiro DY, Okamoto A, et al. Efficacy and safety of tapentadol extended release for the management of chronic low back pain: results of a prospective, randomized, double-blind, placebo- and active-controlled phase III study. Expert Opin Pharmacother. 2010;11(11):1787–804.
Afilalo M, Etropolski MS, Kuperwasser B, et al. Efficacy and safety of tapentadol extended release compared with oxycodone controlled release for the management of moderate to severe chronic pain related to osteoarthritis of the knee: a randomized, double-blind, placebo-and active-controlled phase III study. Clin Drug Investig. 2010;30(8):489–505.
Etropolski M, Kuperwasser B, Flugel M, et al. Safety and tolerability of tapentadol extended release in moderate to severe chronic osteoarthritis or low back pain management: pooled analysis of randomized controlled trials. Adv Ther. 2014;31(6):604–20.
Wild JE, Grond S, Kuperwasser B, et al. Long-term safety and tolerability of tapentadol extended release for the management of chronic low back pain or osteoarthritis pain. Pain Pract. 2010;10(5):416–27.
Grunenthal Ltd. Palexia SR prolonged release tablets: summary of product characteristics. 2017. https://www.medicines.org.uk/. Accessed 30 Oct 2018.
Gohler K, Brett M, Smit JW, et al. Comparative pharmacokinetics and bioavailability of tapentadol following oral administration of immediate- and prolonged-release formulations. Int J Clin Pharmacol Ther. 2013;51(4):338–48.
Huntjens DR, Liefaard LC, Nandy P, et al. Population pharmacokinetic modeling of tapentadol extended release (ER) in healthy subjects and patients with moderate or severe chronic pain. Clin Drug Investig. 2016;36(3):213–23.
Lange B, Kuperwasser B, Okamoto A, et al. Efficacy and safety of tapentadol prolonged release for chronic osteoarthritis pain and low back pain. Adv Ther. 2010;27(6):381–99.
Biondi DM, Xiang J, Etropolski M, et al. Tolerability and efficacy of tapentadol extended release in elderly patients ≥ 75 years of age with chronic osteoarthritis knee or low back pain. J Opioid Manag. 2015;11(5):393–403.
Lange B, Sohns M, Tempero J, et al. Efficacy and safety of tapentadol prolonged release formulation in the treatment of elderly patients with moderate-to-severe chronic osteoarthritis knee pain: a pooled analysis of two double-blind, randomized, placebo-, and active-controlled trials. Curr Med Res Opin. 2018. https://doi.org/10.1080/03007995.2018.1520085.
Baron R, Likar R, Martin-Mola E, et al. Effectiveness of tapentadol prolonged release (PR) compared with oxycodone/naloxone PR for the management of severe chronic low back pain with a neuropathic component: a randomized, controlled, open-label, phase 3b/4 study. Pain Pract. 2016;16(5):580–99.
Baron R, Martin-Mola E, Muller M, et al. Effectiveness and safety of tapentadol prolonged release (PR) versus a combination of tapentadol PR and pregabalin for the management of severe, chronic low back pain with a neuropathic component: a randomized, double-blind, phase 3b study. Pain Pract. 2015;15(5):455–70.
Lange B, von Zabern D, Elling C, et al. Efficacy and safety of tapentadol prolonged release for moderate-to-severe chronic osteoarthritis knee pain: a pooled analysis of two double-blind, randomized, placebo- and oxycodone controlled release-controlled studies. Curr Med Res Opin. 2017;33(8):1413–22.
Sanchez Del Aguila MJ, Schenk M, Kern KU, et al. Practical considerations for the use of tapentadol prolonged release for the management of severe chronic pain. Clin Ther. 2015;37(1):94–113.
Merchant S, Provenzano D, Mody S, et al. Composite measure to assess efficacy/gastrointestinal tolerability of tapentadol ER versus oxycodone CR for chronic pain: pooled analysis of randomized studies. J Opioid Manag. 2013;9(1):51–61.
Buynak R, Rappaport SA, Rod K, et al. Long-term safety and efficacy of tapentadol extended release following up to 2 years of treatment in patients with moderate to severe, chronic pain: results of an open-label extension trial. Clin Ther. 2015;37(11):2420–38.
Etropolski MS, Okamoto A, Shapiro DY, et al. Dose conversion between tapentadol immediate and extended release for low back pain. Pain Physician. 2010;13(1):61–70.
Schwartz S, Etropolski M, Shapiro DY, et al. Safety and efficacy of tapentadol ER in patients with painful diabetic peripheral neuropathy: results of a randomized-withdrawal, placebo-controlled trial. Curr Med Res Opin. 2011;27(1):151–62.
Vinik AI, Shapiro DY, Rauschkolb C, et al. A randomized withdrawal, placebo-controlled study evaluating the efficacy and tolerability of tapentadol extended release in patients with chronic painful diabetic peripheral neuropathy. Diabetes Care. 2014;37(8):2302–9.
Schwartz S, Etropolski MS, Shapiro DY, et al. A pooled analysis evaluating the efficacy and tolerability of tapentadol extended release for chronic, painful diabetic peripheral neuropathy. Clin Drug Investig. 2015;35(2):95–108.
Kress HG, Koch ED, Kosturski H, et al. Tapentadol prolonged release for managing moderate to severe, chronic malignant tumor-related pain. Pain Physician. 2014;17(4):329–43.
Imanaka K, Tominaga Y, Etropolski M, et al. Efficacy and safety of oral tapentadol extended release in Japanese and Korean patients with moderate to severe, chronic malignant tumor-related pain. Curr Med Res Opin. 2013;29(10):1399–409.
Imanaka K, Tominaga Y, Etropolski M, et al. Ready conversion of patients with well-controlled, moderate to severe, chronic malignant tumor-related pain on other opioids to tapentadol extended release. Clin Drug Investig. 2014;34(7):501–11.
Galvez R, Schafer M, Hans G, et al. Tapentadol prolonged release versus strong opioids for severe, chronic low back pain: results of an open-label, phase 3b study. Adv Ther. 2013;30(3):229–59.
Steigerwald I, Schenk M, Lahne U, et al. Effectiveness and tolerability of tapentadol prolonged release compared with prior opioid therapy for the management of severe, chronic osteoarthritis pain. Clin Drug Investig. 2013;33(9):607–19.
Kress HG, Koch ED, Kosturski H, et al. Direct conversion from tramadol to tapentadol prolonged release for moderate to severe, chronic malignant tumour-related pain. Eur J Pain. 2016;20(9):1513–8.
Schwittay A, Schumann C, Litzenburger BC, et al. Tapentadol prolonged release for severe chronic pain: results of a noninterventional study involving general practitioners and internists. J Pain Palliat Care Pharmacother. 2013;27(3):225–34.
Baron R, Jansen JP, Binder A, et al. Tolerability, safety, and quality of life with tapentadol prolonged release (PR) compared with oxycodone/naloxone PR in patients with severe chronic low back pain with a neuropathic component: a randomized, controlled, open-label, phase 3b/4 trial. Pain Pract. 2016;16(5):600–19.
Volkow ND, McLellan AT. Opioid abuse in chronic pain - misconceptions and mitigation strategies. N Engl J Med. 2016;374(13):1253–63.
Centers for Disease Control and Prevention. CDC guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Morb Mortal Wkly Rep. 2016. https://doi.org/10.15585/mmwr.rr6501e1.
Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol. 2012;13(2):e58–68.
American Academy of Pain Medicine. Use of opioids for the treatment of chronic pain. 2013. http://www.painmed.org. Accessed 30 Oct 2018.
Leppert W. The impact of opioid analgesics on the gastrointestinal tract function and the current management possibilities. Contemp Oncol (Pozn). 2012;16(2):125–31.
Salwan AJ, Hagemeier NE, Harirforoosh S. Abuse-deterrent opioid formulations: a key ingredient in the recipe to prevent opioid disasters? Clin Drug Investig. 2018;38(7):573–7.
Vosburg SK, Severtson SG, Dart RC, et al. Assessment of tapentadol API abuse liability with the Researched Abuse, Diversion and Addiction-Related Surveillance System. J Pain. 2018;19(4):439–53.
Baron R, Kern U, Muller M, et al. Effectiveness and tolerability of a moderate dose of tapentadol prolonged release for managing severe, chronic low back pain with a neuropathic component: an open-label continuation arm of a randomized phase 3b study. Pain Pract. 2015;15(5):471–86.
Acknowledgements
During the peer review process, the manufacturer of tapentadol PR was also offered the opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflicts of interest
Emma Deeks is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
The manuscript was reviewed by: C. T. Hartrick, Department of Biomedical Sciences, Oakland University William Beaumont School of Medicine, Rochester, MI, USA; R. Sabatowski, Pain Center, University Hospital Carl Gustav Carus, Technical University Dresden, Dresden, Germany.
The original version of this article was revised due to a retrospective Open Access request.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
About this article
Cite this article
Deeks, E.D. Tapentadol Prolonged Release: A Review in Pain Management. Drugs 78, 1805–1816 (2018). https://doi.org/10.1007/s40265-018-1007-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-018-1007-2