Abstract
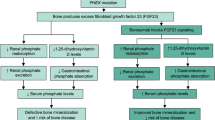
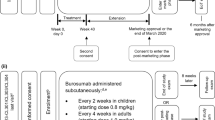
Burosumab (Crysvita®; Kyowa Hakko Kirin Co., Ltd. and Ultragenyx Pharmaceutical Inc.) is a fully human monoclonal antibody directed at fibroblast growth factor 23 (FGF23). Excessive FGF23 production has been implicated in various hypophosphataemic diseases. Inhibition of FGF23 by burosumab results in increased renal phosphate reabsorption and increased serum levels of phosphorus and active vitamin D. In February 2018, the EMA granted subcutaneous burosumab conditional marketing authorization for the treatment of X-linked hypophosphataemia (XLH) with radiographic evidence of bone disease in children one year of age and older and adolescents with growing skeletons. In April 2018, the US FDA approved burosumab for the treatment of XLH in adults and children one year of age and older. Multinational phase III trials of burosumab are currently underway in adult and paediatric patients with XLH. Burosumab is also being evaluated in the phase II setting in adults with tumour-induced osteomalacia and epidermal nevus syndrome in the USA, as well as in Japan and Korea. This article summarizes the milestones in the development of burosumab leading to its first global approval in the EU for XLH in paediatric patients.
Similar content being viewed by others
References
Ruppe MD. GeneReviews®: X-linked hypophosphatemia. 2017. https://www.ncbi.nlm.nih.gov/. Accessed 6 Apr 2018.
Beck-Nielsen SS, Brock-Jacobsen B, Gram J, et al. Incidence and prevalence of nutritional and hereditary rickets in southern Denmark. Eur J Endocrinol. 2009;160:491–7.
Shore RM, Chesney RW. Rickets: part II. Pediatr Radiol. 2013;43(2):152–72.
Endo I, Fukumoto S, Ozono K, et al. Nationwide survey of fibroblast growth factor 23 (FGF23)-related hypophosphatemic diseases in Japan: prevalence, biochemical data and treatment. Endocr J. 2015;62(9):811–6.
Carpenter TO. New perspectives on the biology and treatment of X-linked hypophosphatemic rickets. Pediatr Clin N Am. 1997;44(2):443–66.
Zhang X, Imel EA, Ruppe MD, et al. Pharmacokinetics and pharmacodynamics of a human monoclonal anti-FGF23 antibody (KRN23) in the first multiple ascending-dose trial treating adults with X-linked hypophosphatemia. J Clin Pharmacol. 2016;56(2):176–85.
Zhang X, Peyret T, Gosselin NH, et al. Population pharmacokinetic and pharmacodynamic analyses from a 4-month intradose escalation and its subsequent 12-month dose titration studies for a human monoclonal anti-FGF23 antibody (KRN23) in adults with X-linked hypophosphatemia. J Clin Pharmacol. 2016;56(4):429–38.
European Medicines Agency. CRYSVITA (Burosumab): summary of product characteristics. 2018. http://ec.europa.eu/. Accessed 4 Apr 2018.
Ultragenyx Pharmaceutical Inc. Kyowa Kirin and Ultragenyx announce Crysvita® (burosumab) receives conditional marketing authorization in Europe for the treatment of X-linked hypophosphatemia in children. [media release]. 23 Feb 2018. http://ir.ultragenyx.com/.
European Medicines Agency. New medicine for rare bone disease: Crysvita, a medicine for the treatment of X-linked hypophosphataemia, recommended for conditional approval. 2017. http://www.ema.europa.eu/. Accessed 4 Apr 2018.
European Medicines Agency. Human medicines highlights 2017. 2018. http://www.ema.europa.eu/. Accessed 6 Apr 2018.
US FDA. FDA approves first therapy for rare inherited form of rickets, x-linked hypophosphatemia. [media release]. 17 Apr 2018. http://www.fda.gov/
Ultragenyx Pharmaceutical, Kyowa Hakko Kirin. Ultragenyx and Kyowa Hakko Kirin announce FDA acceptance and priority review designation of burosumabs biologics license application. [media release]. 10 Oct 2017. http://www.ultragenyx.com.
Ultragenyx Pharmaceutical. Ultragenyx granted EU orphan drug designation for KRN23 for the treatment of X-linked hypophosphatemia. [media release]. 30 Oct 2014. http://www.ultragenyx.com.
Ultragenyx Pharmaceutical, Inc. Ultragenyx announces collaboration with Kyowa Hakko Kirin to develop and commercialize phase 2-stage KRN23 for X-linked hypophosphatemia. [media release]. 3 Sep 2013. http://www.ultragenyx.com.
Ultragenyx Pharmaceutical Inc. Form 10-Q. 2017. http://ir.ultragenyx.com/. Accessed 4 Apr 2018.
Ultragenyx Pharmaceutical. Ultragenyx reports second quarter 2017 financial results and corporate update 2017. http://ir.ultragenyx.com/. Accessed 4 Apr 2018.
European Medicines Agency. CHMP assessment report: Crysvita (burosumab). 2017. http://www.ema.europa.eu/. Accessed 4 Apr 2018.
Aono Y, Yamazaki Y, Yasutake J, et al. Therapeutic effects of anti-FGF23 antibodies in hypophosphatemic rickets/osteomalacia. J Bone Miner Res. 2009;24:1879–88.
Carpenter TO, Imel EA, Ruppe MD, et al. Randomized trial of the anti-FGF23 antibody KRN23 in X-linked hypophosphatemia. J Clin Invest. 2014;124(4):1587–97.
Ultragenyx Pharmaceutical. Ultragenyx, Kyowa Hakko Kirin and Kyowa Kirin International announce positive 24-week data from adult phase 3 study of burosumab (KRN23) in X-linked hypophosphatemia. [media release]. 18 Apr 2017. http://www.ultragenyx.com.
Ultragenyx Pharmaceutical, Kyowa Kirin International, Kyowa Hakko Kirin. Ultragenyx and Kyowa Kirin announce positive 48-week data from adult phase 3 study of burosumab (KRN23) in X-linked hypophosphatemia. [media release]. 4 Dec 2017. http://www.ultragenyx.com.
Imel EA, Zhang X, Ruppe MD, et al. Prolonged correction of serum phosphorus in adults with X-linked hypophosphatemia using monthly doses of KRN23. J Clin Endocrinol Metab. 2015;100(7):2565–73.
Ruppe M, Peacock M, Weber T, et al. Clinical and radiographic characteristics of adult X-linked hypophosphatemia (XLH) in a cohort of patients treated with KRN23, an antibody to FGF23 [abstract no. MO0319]. In: 38th Annual Meeting of the American Society for Bone and Mineral Research. 2016.
Carpenter T, Miller PD, Weber T, et al. Effects of KRN23, an anti-FGF23 antibody, in patients with tumor-induced osteomalacia or epidermal nevus syndrome-associated osteomalacia: Interim results from a phase 2 study [abstract no. P531]. Osteoporos Int. 2017;28(Suppl 1):S337–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the author on the basis of scientific completeness and accuracy. Yvette Lamb is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Lamb, Y.N. Burosumab: First Global Approval. Drugs 78, 707–714 (2018). https://doi.org/10.1007/s40265-018-0905-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-018-0905-7