Abstract
Background and Aims
Ezetimibe reduces plasma low-density lipoprotein cholesterol (LDL-C) levels by up to 20%. However, its effect on plasma lipoprotein(a) [Lp(a)] concentrations in patients with primary hypercholesterolemia has not been defined.
Objective
Therefore, we performed a systematic review and meta-analysis to assess this effect based on the available randomized controlled trials (RCTs).
Methods
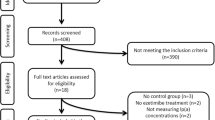
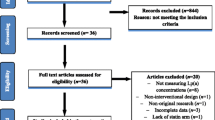
We searched the PubMed and SCOPUS databases from inception until 28 February 2017 to identify RCTs that investigated the effect of ezetimibe monotherapy on plasma Lp(a) concentrations in patients with primary hypercholesterolemia. We pooled mean percentage changes in plasma Lp(a) concentrations as a mean difference (MD) with a 95% confidence interval (CI).
Results
Seven RCTs with 2337 patients met the selection criteria and were included in the analysis. Overall pooled analysis suggested that ezetimibe 10 mg significantly reduced plasma Lp(a) concentrations in patients with primary hypercholesterolemia by − 7.06% (95% CI − 11.95 to − 2.18; p = 0.005) compared with placebo. No significant heterogeneity was observed (χ2 = 5.34; p = 0.5). Excluding one study from the analysis resulted in insignificant differences between the two groups (p = 0.2). Meta-regression did not find a significant association between the mean percentage changes in Lp(a) and other potential moderator variables, which included the mean percentage changes of LDL-C concentrations (p = 0.06) and baseline Lp(a) mean values (p = 0.46).
Conclusions
Ezetimibe monotherapy (10 mg/day) showed a small (7.06%) but statistically significant reduction in the plasma levels of Lp(a) in patients with primary hypercholesterolemia. According to current literature, this magnitude of reduction seems to have no clinical relevance. However, further studies are warranted to clarify the mechanism mediating this effect of ezetimibe and to investigate its efficacy in combination with other drugs that have shown promise in lowering Lp(a) levels.



Similar content being viewed by others
References
Berg K. A new serum type system in man—the Lp system. Acta Pathol Microbiol Scand. 1963;59:369–82.
Hoover-Plow J, Huang M. Lipoprotein(a) metabolism: potential sites for therapeutic targets. Metabolism. 2013;62:479–91.
Banach M. Lipoprotein(a)—we know so much yet still have much to learn …. J. Am. Heart Assoc. 2016;5:e003597.
Kronenberg F, Steinmetz A, Kostner GM, Dieplinger H. Lipoprotein(a) in health and disease. Crit Rev Clin Lab Sci. 1996;33:495–543.
McLean JW, Tomlinson JE, Kuang W-J, Eaton DL, Chen EY, Fless GM, et al. cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature. 1987;330:132–7.
Marcovina SM, Koschinsky ML, Albers JJ, Skarlatos S. Report of the National Heart, Lung, and Blood Institute workshop on lipoprotein(a) and cardiovascular disease: recent advances and future directions. Clin Chem. 2003;49:1785–96.
Bermúdez V, Arráiz N, Aparicio D, Rojas E, Gotera D, Guerra X, et al. Lipoprotein(a): from molecules to therapeutics. Am J Ther. 2010;17:263–73.
Kotani K, Serban M-C, Penson P, Lippi G, Banach M. Evidence-based assessment of lipoprotein(a) as a risk biomarker for cardiovascular diseases – Some answers and still many questions. Crit Rev Clin Lab Sci. 2016;53:370–8.
Kotani K, Banach M. Lipoprotein(a) and inhibitors of proprotein convertase subtilisin/kexin type 9. J Thorac Dis. 2017;9:E78–82.
White AL, Guerra B, Lanford RE. Influence of allelic variation on apolipoprotein(a) folding in the endoplasmic reticulum. J Biol Chem. 1997;272:5048–55.
Lippi G, Guidi G. Lipoprotein(a): from ancestral benefit to modern pathogen? QJM. 2000;93:75–84.
McCormick SPA. Lipoprotein(a): biology and clinical importance. Clin Biochem Rev. 2004;25:69–80.
Pirro M, Bianconi V, Paciullo F, Mannarino MR, Bagaglia F, Sahebkar A. Lipoprotein(a) and inflammation: a dangerous duet leading to endothelial loss of integrity. Pharmacol Res. 2017;119:178–87.
Orsó E, Schmitz G. Lipoprotein(a) and its role in inflammation, atherosclerosis and malignancies. Clin Res Cardiol Suppl. 2017;12:31–7.
Leibundgut G, Scipione C, Yin H, Schneider M, Boffa MB, Green S, et al. Determinants of binding of oxidized phospholipids on apolipoprotein(a) and lipoprotein(a). J Lipid Res. 2013;54:2815–30.
Hajjar MDKA, Nachman MDRL. The role of lipoprotein(a) in atherogenesis and thrombosis. Annu Rev Med. 1996;47:423–42.
Rader DJ, Hoeg JM, Brewer HB. Quantitation of plasma apolipoproteins in the primary and secondary prevention of coronary artery disease. Ann Intern Med. 1994;120:1012–25.
Nordestgaard BG, Langsted A. Lipoprotein(a) as a cause of cardiovascular disease: insights from epidemiology, genetics, and biology. J Lipid Res. 2016;57:1953–75.
Ezhov MV, Safarova MS, Afanasieva OI, Kukharchuk VV, Pokrovsky SN. Lipoprotein(a) level and apolipoprotein(a) phenotype as predictors of long-term cardiovascular outcomes after coronary artery bypass grafting. Atherosclerosis. 2014;235:477–82.
Kotani K, Sahebkar A, Serban M-C, Ursoniu S, Mikhailidis DP, Mariscalco G, et al. Lipoprotein(a) levels in patients with abdominal aortic aneurysm. Angiology. 2017;68:99–108.
Bozbaş H, Yildirir A, Pirat B, Eroğlu S, Korkmaz ME, Atar I, et al. Increased lipoprotein(a) in metabolic syndrome: is it a contributing factor to premature atherosclerosis? Anadolu Kardiyol Derg. 2008;8:111–5.
Sung K-C, Wild SH, Byrne CD. Lipoprotein(a), metabolic syndrome and coronary calcium score in a large occupational cohort. Nutr Metab Cardiovasc Dis. 2013;23:1239–46.
Yun J-S, Ahn Y-B, Song K-H, Yoo K-D, Park Y-M, Kim H-W, et al. Lipoprotein(a) predicts a new onset of chronic kidney disease in people with Type 2 diabetes mellitus. Diabet Med. 2016;33:639–43.
Franchini M, Capuzzo E, Liumbruno GM. Lipoprotein apheresis for the treatment of elevated circulating levels of lipoprotein(a): a critical literature review. Blood Transfus. 2016;14:413–8.
Sahebkar A, Reiner Ž, Simental-Mendía LE, Ferretti G, Cicero AFG. Effect of extended-release niacin on plasma lipoprotein(a) levels: a systematic review and meta-analysis of randomized placebo-controlled trials. Metabolism. 2016;65:1664–78.
Panta R, Dahal K, Kunwar S. Efficacy and safety of mipomersen in treatment of dyslipidemia: a meta-analysis of randomized controlled trials. J Clin Lipidol. 2015;9:217–25.
Zhang X-L, Zhu Q-Q, Zhu L, Chen J-Z, Chen Q-H, Li G-N, et al. Safety and efficacy of anti-PCSK9 antibodies: a meta-analysis of 25 randomized, controlled trials. BMC Med. 2015;13:123.
Ballantyne CM, Shah S, Kher U, Hunter JA, Gill GG, Cressman MD, et al. Lipid-modifying efficacy and tolerability of anacetrapib added to ongoing statin therapy in patients with hypercholesterolemia or low high-density lipoprotein cholesterol. Am J Cardiol. 2017;119:388–96.
Viney NJ, van Capelleveen JC, Geary RS, Xia S, Tami JA, Yu RZ, et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet. 2016;388:2239–53.
Altmann SW, Davis HR, Zhu L-J, Yao X, Hoos LM, Tetzloff G, et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–4.
Weinglass AB, Kohler M, Schulte U, Liu J, Nketiah EO, Thomas A, et al. Extracellular loop C of NPC1L1 is important for binding to ezetimibe. Proc Natl Acad Sci USA. 2008;105:11140–5.
Miura S, Saku K. Beneficial effects of ezetimibe-based therapy in patients with dyslipidemia. J Cardiol. 2008;52:1–6.
Tobaru T, Seki A, Asano R, Sumiyoshi T, Hagiwara N. Lipid-lowering and anti-inflammatory effect of ezetimibe in hyperlipidemic patients with coronary artery disease. Heart Vessels. 2013;28:39–45.
Tie C, Gao K, Zhang N, Zhang S, Shen J, Xie X, et al. Ezetimibe attenuates atherosclerosis associated with lipid reduction and inflammation inhibition. PLoS One. 2015;10:e0142430.
Qin L, Yang Y-B, Yang Y-X, Zhu N, Li S-X, Liao D-F, et al. Anti-Inflammatory activity of ezetimibe by regulating NF-κB/MAPK pathway in THP-1 macrophages. Pharmacology. 2014;93:69–75.
Gómez-Garre D, Muñoz-Pacheco P, González-Rubio M, Aragoncillo P, Granados R, Fernández-Cruz A. Ezetimibe reduces plaque inflammation in a rabbit model of atherosclerosis and inhibits monocyte migration in addition to its lipid-lowering effect. Br J Pharmacol. 2009;156:1218–27.
Noma A, Abe A, Maeda S, Seishima M, Makino K, Yano Y, et al. Lp(a): an acute-phase reactant? Chem Phys Lipids. 1994;67–68:411–7.
Wade DP, Clarke JG, Lindahl GE, Liu AC, Zysow BR, Meer K, et al. 5′ control regions of the apolipoprotein(a) gene and members of the related plasminogen gene family. Proc Natl Acad Sci USA. 1993;90:1369–73.
Wang J, Hu B, Kong L, Cai H, Zhang C. Native, oxidized lipoprotein(a) and lipoprotein(a) immune complex in patients with active and inactive rheumatoid arthritis: plasma concentrations and relationship to inflammation. Clin Chim Acta. 2008;390:67–71.
Crook MA, Haq S, Chusney G, Haq M, Tutt P. Acute phase proteins and lipoprotein(a) in patients with severe hypercholesterolaemia and normal subjects. Clin Chim Acta. 1994;224:199–201.
Nozue T, Michishita I, Mizuguchi I. Effects of ezetimibe on remnant-like particle cholesterol, lipoprotein(a), and oxidized low-density lipoprotein in patients with dyslipidemia. J Atheroscler Thromb. 2010;17:37–44.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Higgins J, Green S. Cochrane handbook for systematic reviews of interventions. 2011. https://www.google.com/books?hl=en&lr=&id=NKMg9sMM6GUC&oi=fnd&pg=PT13&dq=Cochrane+Handbook+for+Systematic+Reviews+of+Interventions&ots=LH_DUZIDB-&sig=ua2m0uDvgBT23R-Brd0IVYbaY-4. Accessed 20 Nov 2017.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Barendregt J, Doi S. MetaXL User Guide. http://www.epigear.com/index_files/MetaXL. Accessed 20 Nov 2017. User Guide.pdf.
Ballantyne CM, Houri J, Notarbartolo A, Melani L, Lipka LJ, Suresh R, et al. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation. 2003;107:2409–15.
Davidson MH, McGarry T, Bettis R, Melani L, Lipka LJ, LeBeaut AP, et al. Ezetimibe coadministered with simvastatin in patients with primary hypercholesterolemia. J Am Coll Cardiol. 2002;40:2125–34.
Dujovne CA, Ettinger MP, McNeer JF, Lipka LJ, LeBeaut AP, Suresh R, et al. Efficacy and safety of a potent new selective cholesterol absorption inhibitor, ezetimibe, in patients with primary hypercholesterolemia. Am J Cardiol. 2002;90:1092–7.
Goldberg AC, Sapre A, Liu J, Capece R, Mitchel YB. Ezetimibe study group. efficacy and safety of ezetimibe coadministered with simvastatin in patients with primary hypercholesterolemia: a randomized, double-blind, placebo-controlled trial. Mayo Clin Proc. 2004;79:620–9.
Kerzner B, Corbelli J, Sharp S, Lipka LJ, Melani L, LeBeaut A, et al. Efficacy and safety of ezetimibe coadministered with lovastatin in primary hypercholesterolemia. Am J Cardiol. 2003;91:418–24.
Knopp RH, Gitter H, Truitt T, Bays H, Manion CV, Lipka LJ, et al. Effects of ezetimibe, a new cholesterol absorption inhibitor, on plasma lipids in patients with primary hypercholesterolemia. Eur Heart J. 2003;24:729–41.
Melani L, Mills R, Hassman D, Lipetz R, Lipka L, LeBeaut A, et al. Efficacy and safety of ezetimibe coadministered with pravastatin in patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Eur Heart J. 2003;24:717–28.
Xu M-X, Liu C, He Y-M, Yang X-J, Zhao X. Long-term statin therapy could be efficacious in reducing the lipoprotein(a) levels in patients with coronary artery disease modified by some traditional risk factors. J Thorac Dis. 2017;9:1322–32.
Kostakou P, Kolovou G, Anagnostopoulou K, Theodoridis T, Galea V, Mihas C, et al. Efficacy of simvastatin or ezetimibe on tissue factor, von Willebrand’s factor and C-reactive protein in patients with hypercholesterolaemia. Arch Cardiovasc Dis. 2010;103:26–32.
Morita T, Morimoto S, Nakano C, Kubo R, Okuno Y, Seo M, et al. Renal and vascular protective effects of ezetimibe in chronic kidney disease. Intern Med. 2014;53:307–14.
Bays HE, Moore PB, Drehobl MA, Rosenblatt S, Toth PD, Dujovne CA, et al. Effectiveness and tolerability of ezetimibe in patients with primary hypercholesterolemia: pooled analysis of two phase II studies. Clin Ther. 2001;23:1209–30.
Geiss HC, Otto C, Hund-Wissner E, Parhofer KG. Effects of ezetimibe on plasma lipoproteins in severely hypercholesterolemic patients treated with regular LDL-apheresis and statins. Atherosclerosis. 2005;180:107–12.
Albers JJ, Slee A, O’Brien KD, Robinson JG, Kashyap ML, Kwiterovich PO, et al. Relationship of apolipoproteins A-1 and B, and lipoprotein(a) to cardiovascular outcomes. J Am Coll Cardiol. 2013;62:1575–9.
Wierzbicki AS, Doherty E, Lumb PJ, Chik G, Crook MA. Efficacy of ezetimibe in patients with statin-resistant and statin-intolerant familial hyperlipidaemias. Curr Med Res Opin. 2005;21:333–8.
McKenney JM, Jones PH, Bays HE, Knopp RH, Kashyap ML, Ruoff GE, et al. Comparative effects on lipid levels of combination therapy with a statin and extended-release niacin or ezetimibe versus a statin alone (the COMPELL study). Atherosclerosis. 2007;192:432–7.
Pitsavos C, Skoumas I, Tousoulis D, Metalinos G, Masoura C, Chrysohoou C, et al. The impact of ezetimibe and high-dose of statin treatment on LDL levels in patients with heterozygous familial hypercholesterolemia. Int J Cardiol. 2009;134:280–1.
Yeang C, Hung M-Y, Byun Y-S, Clopton P, Yang X, Witztum JL, et al. Effect of therapeutic interventions on oxidized phospholipids on apolipoprotein B100 and lipoprotein(a). J Clin Lipidol. 2016;10:594–603.
Sahebkar A, Simental-Mendía LE, Watts GF, Serban M-C, Banach M. Lipid and Blood Pressure Meta-analysis Collaboration (LBPMC) Group L and BPMC (LBPMC). Comparison of the effects of fibrates versus statins on plasma lipoprotein(a) concentrations: a systematic review and meta-analysis of head-to-head randomized controlled trials. BMC Med. 2017;15:22.
Gaudet D, Watts GF, Robinson JG, Minini P, Sasiela WJ, Edelberg J, et al. Effect of alirocumab on lipoprotein(a) over ≥ 1.5 years (from the Phase 3 ODYSSEY Program). Am J Cardiol. 2017;119:40–6.
Rey J, Poitiers F, Paehler T, Brunet A, DiCioccio AT, Cannon CP, et al. Relationship between low-density lipoprotein cholesterol, free proprotein convertase subtilisin/kexin type 9, and alirocumab levels after different lipid-lowering strategies. J Am Heart Assoc. 2016;5:e003323.
Zetia approval letter. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/21445_Zetia_Approv.pdf. Accessed 20 Nov 2017.
Funding
No external funding was used in the conduct of this work.
Author information
Authors and Affiliations
Consortia
Contributions
KA contributed to the literature search, screening, data extraction, data analysis, and manuscript writing; DPM to the manuscript writing and revision; NK to the screening, data extraction, and manuscript writing; PM to the manuscript writing and revision; and MB to the study design, supervision, coordination and manuscript writing, and revision. All authors approved the final article.
Corresponding author
Ethics declarations
Conflict of interest
DPM has given talks and attended conferences sponsored by MSD, AstraZeneca, and Libytec. NK has given talks, attended conferences, and participated in trials sponsored by Amgen, Angelini, Astra Zeneca, Boehringer Ingelheim, MSD, Novartis, NovoNordisk, Sanofi, and WinMedica. PM has received grant support and honoraria from Amgen. MB has received advisory board fees from Abbott Vascular, Amgen, Daichi Sankyo, Esperion, Lilly, MSD, Resverlogix, and Sanofi-Aventis; speakers bureau fees from Abbott/Mylan, Abbott Vascular, Actavis, Akcea, Amgen, Biofarm, KRKA, MSD, Sanofi-Aventis, and Valeant; and grants from Valeant and Sanofi-Aventis. KA has no conflicts of interest that are directly relevant to the content of this review.
Electronic supplementary material
Below is the link to the electronic supplementary material.
40265_2018_870_MOESM4_ESM.tif
Supplementary figure 2 The Doi plot displaying publication bias in the studies reporting the impact of ezetimibe monotherapy on plasma Lp(a) concentrations in patients with primary hypercholesterolemia. WMD weighted mean difference
Rights and permissions
About this article
Cite this article
Awad, K., Mikhailidis, D.P., Katsiki, N. et al. Effect of Ezetimibe Monotherapy on Plasma Lipoprotein(a) Concentrations in Patients with Primary Hypercholesterolemia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Drugs 78, 453–462 (2018). https://doi.org/10.1007/s40265-018-0870-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-018-0870-1